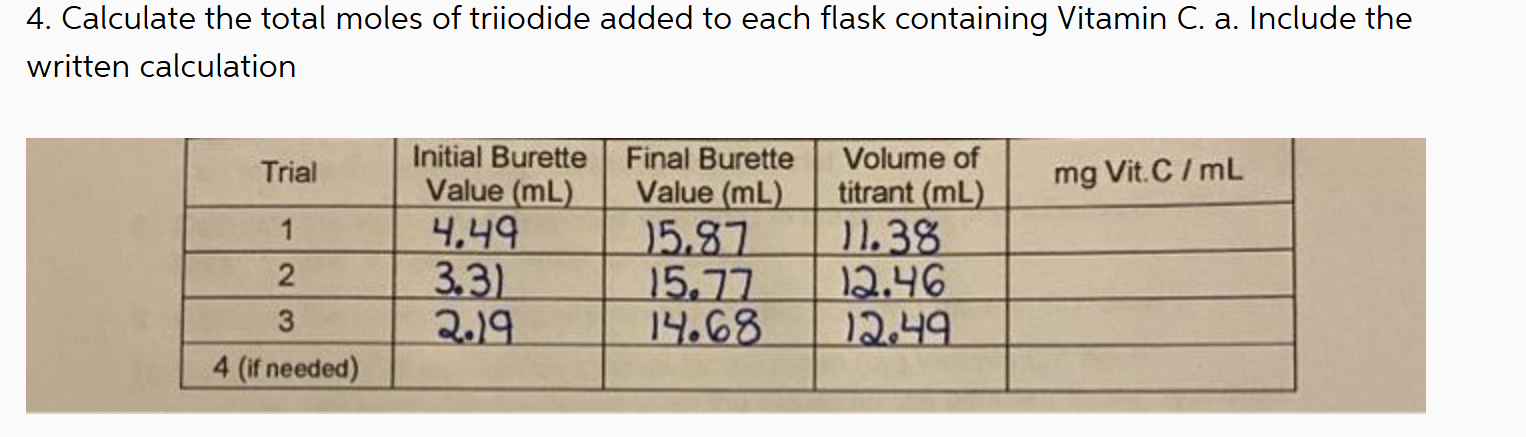
Calculate the total moles of triiodide added to each flask containing Vitamin C. Include the written calculation.
Understand the Problem
The question asks to calculate the total moles of triiodide added to each flask containing Vitamin C, using the provided titration data including initial and final burette values for three trials. We'll need to use the volume of titrant (triiodide) and its concentration (which is missing, we need to look it up or assume it is given elsewhere) convert volume to moles for each trial.
Answer
Assuming the concentration of triiodide is 0.01 M: Trial 1: $0.0001138 \text{ moles}$ Trial 2: $0.0001246 \text{ moles}$ Trial 3: $0.0001249 \text{ moles}$
Answer for screen readers
Assuming the concentration of triiodide is 0.01 M: Trial 1: $0.0001138 \text{ moles}$ Trial 2: $0.0001246 \text{ moles}$ Trial 3: $0.0001249 \text{ moles}$
Steps to Solve
- Identify the given data
We have the volume of titrant from three trials: Trial 1: 11.38 mL Trial 2: 12.46 mL Trial 3: 12.49 mL
- Find the concentration of the triiodide solution
The concentration of the triiodide solution is needed to calculate the number of moles. Since it is not provided in the text, we will assume a concentration of 0.01 M (mol/L) for demonstration purposes. In a real experiment, this value would be known.
- Convert volume from mL to L
To convert mL to L, divide by 1000: Trial 1: $11.38 \text{ mL} \times \frac{1 \text{ L}}{1000 \text{ mL}} = 0.01138 \text{ L}$ Trial 2: $12.46 \text{ mL} \times \frac{1 \text{ L}}{1000 \text{ mL}} = 0.01246 \text{ L}$ Trial 3: $12.49 \text{ mL} \times \frac{1 \text{ L}}{1000 \text{ mL}} = 0.01249 \text{ L}$
- Calculate the moles of triiodide for each trial
Use the formula: $\text{moles} = \text{concentration} \times \text{volume}$ Trial 1: $\text{moles} = 0.01 \text{ M} \times 0.01138 \text{ L} = 0.0001138 \text{ moles}$ Trial 2: $\text{moles} = 0.01 \text{ M} \times 0.01246 \text{ L} = 0.0001246 \text{ moles}$ Trial 3: $\text{moles} = 0.01 \text{ M} \times 0.01249 \text{ L} = 0.0001249 \text{ moles}$
- Summarize the results
Total moles of triiodide added: Trial 1: 0.0001138 moles Trial 2: 0.0001246 moles Trial 3: 0.0001249 moles
Assuming the concentration of triiodide is 0.01 M: Trial 1: $0.0001138 \text{ moles}$ Trial 2: $0.0001246 \text{ moles}$ Trial 3: $0.0001249 \text{ moles}$
More Information
The number of moles of triiodide is directly proportional to the volume of the titrant used, given a fixed concentration of the triiodide solution. The assumption of 0.01M is only for demonstration, the actual concentration of the triiodide solution is needed to calculate the real number of moles.
Tips
A common mistake is forgetting to convert the volume from mL to L before calculating the number of moles. Also, using the wrong concentration of the triiodide solution or not having the concentration at all will result in a wrong answer.
AI-generated content may contain errors. Please verify critical information