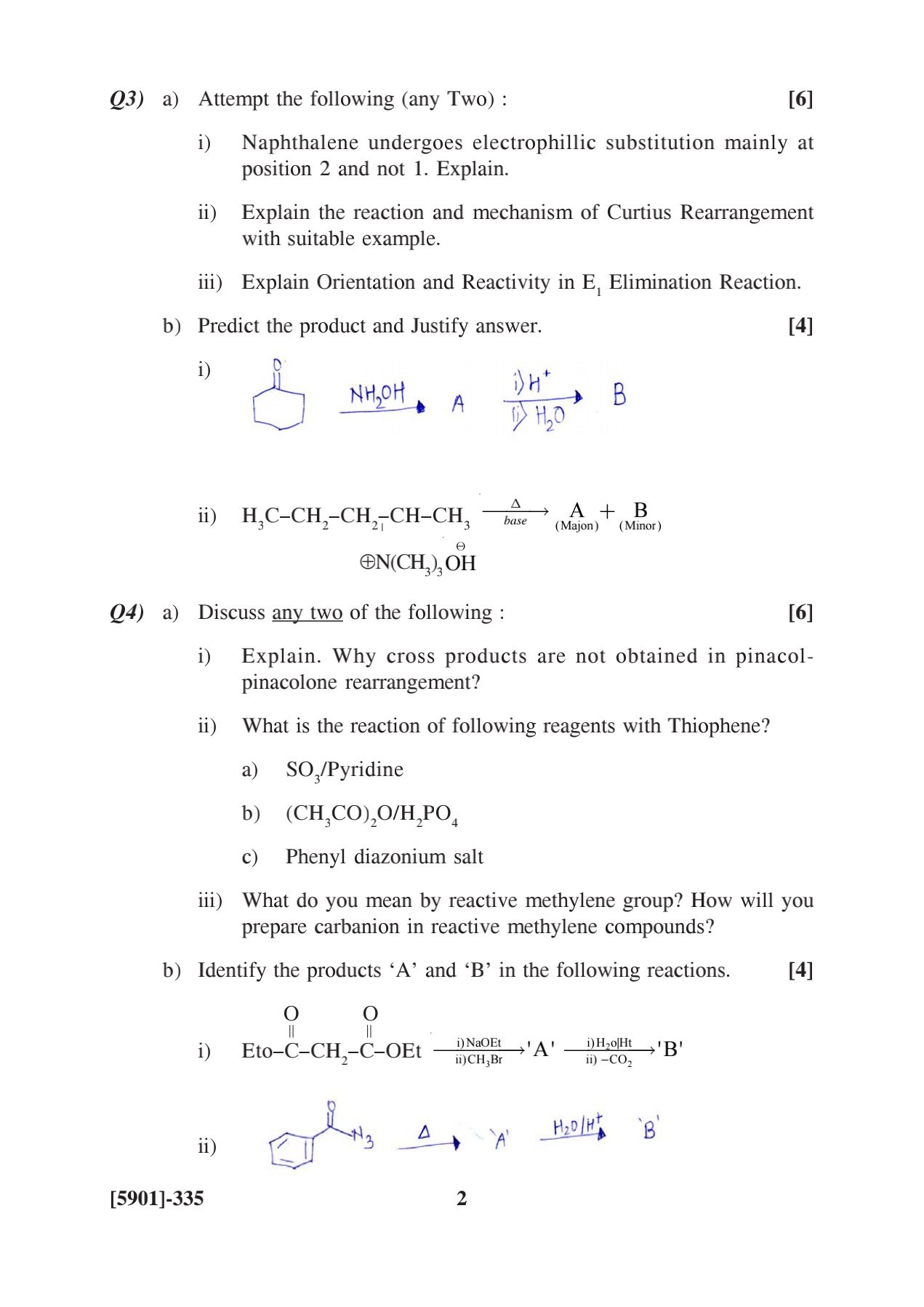
Attempt the following (any two): i) Naphthalene undergoes electrophilic substitution mainly at position 2 and not 1. Explain. ii) Explain the reaction and mechanism of Curtius Rear... Attempt the following (any two): i) Naphthalene undergoes electrophilic substitution mainly at position 2 and not 1. Explain. ii) Explain the reaction and mechanism of Curtius Rearrangement with suitable example. iii) Explain Orientation and Reactivity in E1 Elimination Reaction. Predict the product and Justify answer. Discuss any two of the following: i) Explain why cross products are not obtained in pinacolone rearrangement? ii) What is the reaction of following reagents with Thiophene? a) SO3/Pyridine b) (CH3CO)2O/H2PO4 c) Phenyl diazonium salt iii) What do you mean by reactive methylene group? How will you prepare carbocation in reactive methylene compounds? Identify the products ‘A’ and ‘B’ in the following reactions.
Understand the Problem
The question is asking to discuss various organic chemistry concepts such as electrophilic substitution in naphthalene, Curtius rearrangement, E1 elimination reactions, and reactions involving thiophene. It also asks to identify products in given reactions, showing an understanding of mechanisms and product outcomes.
Answer
Naphthalene substitutes at position 2 due to stability; Curtius rearrangement forms amines; E1 favors more stable alkenes.
Naphthalene prefers substitution at position 2 due to its more stable intermediate. Curtius rearrangement converts acyl azides to isocyanates, forming amines. E1 elimination favors more substituted alkenes.
Answer for screen readers
Naphthalene prefers substitution at position 2 due to its more stable intermediate. Curtius rearrangement converts acyl azides to isocyanates, forming amines. E1 elimination favors more substituted alkenes.
More Information
Electrophilic substitution at position 2 in naphthalene is preferred because it results in more resonance-stabilized intermediates, making the reaction more favorable.
Tips
A common mistake in E1 reactions is neglecting the possibility of carbocation rearrangement, which can lead to unexpected major products.
Sources
- Substitution Reactions of Polynuclear Aromatic Hydrocarbons - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information