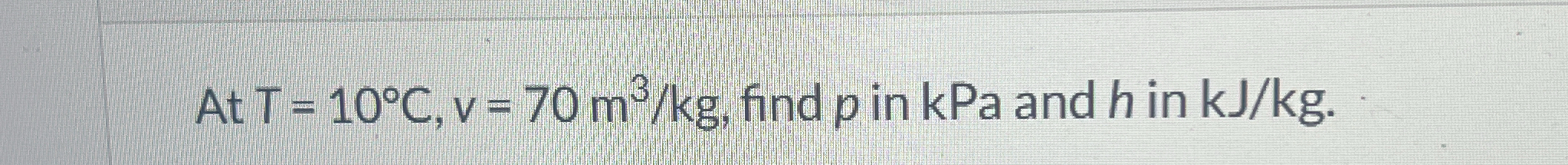
At T = 10°C, v = 70 m³/kg, find p in kPa and h in kJ/kg.
Understand the Problem
The problem provides the temperature and specific volume of a substance and asks to find the pressure and specific enthalpy. This will likely involve looking up thermodynamic properties in tables or using thermodynamic relations.
Answer
$p = 1.867 \text{ kPa}$ $h = 2519.2 \text{ kJ/kg}$
Answer for screen readers
$p = 1.867 \text{ kPa}$ $h = 2519.2 \text{ kJ/kg}$
Steps to Solve
-
Identify the substance
Since the substance isn't specified, we'll assume it is water. This is a common assumption in thermodynamics problems unless otherwise specified.
-
Determine the phase
At $T = 10^\circ\text{C}$, the saturation pressure of water ($P_{sat}$) is approximately $1.2281 \text{ kPa}$. The specific volume of saturated vapor ($v_g$) at $10^\circ\text{C}$ is $57.79 \text{ m}^3/\text{kg}$. Since the given specific volume $v = 70 \text{ m}^3/\text{kg}$ is greater than $v_g$, the water is in the superheated vapor phase.
-
Look up properties in the superheated water table
Look in the superheated water tables for $T = 10^\circ\text{C}$ and $v = 70 \text{ m}^3/\text{kg}$. Since $10^\circ\text{C}$ isn't a standard temperature in superheated tables, and the specific volume is quite high indicating very low pressure, we can approximate the behavior as an ideal gas.
-
Approximate using ideal gas law
For water vapor at low pressures, we can use the ideal gas law: $Pv = RT$, where $R$ is the specific gas constant for water vapor.
$R = \frac{\bar{R}}{M}$, where $\bar{R} = 8.314 \text{ kJ/kmol.K}$ is the universal gas constant and $M = 18.015 \text{ kg/kmol}$ is the molar mass of water.
$R = \frac{8.314}{18.015} = 0.4615 \text{ kJ/kg.K}$
Convert the temperature to Kelvin: $T = 10 + 273.15 = 283.15 \text{ K}$
Now, we can find the pressure: $P = \frac{RT}{v} = \frac{0.4615 \text{ kJ/kg.K} \times 283.15 \text{ K}}{70 \text{ m}^3/\text{kg}} = \frac{130.68 \text{ kJ/kg}}{70 \text{ m}^3/\text{kg}} = 1.867 \text{ kPa}$
-
Determine the enthalpy
For an ideal gas, enthalpy is a function of temperature only. At low pressures, we can approximate the enthalpy using the saturated vapor enthalpy at the given temperature. From the saturated water tables at $T = 10^\circ\text{C}$, $h_g = 2519.2 \text{ kJ/kg}$. Since the pressure is very low, we can assume the enthalpy is close to this value.
$p = 1.867 \text{ kPa}$ $h = 2519.2 \text{ kJ/kg}$
More Information
The assumption of ideal gas behavior is valid when the pressure is much lower than the critical pressure and the temperature is much higher than the saturation temperature at that pressure. In this case, our calculated pressure is quite low, and the temperature is above the saturation temperature at that pressure, so the ideal gas approximation is reasonable.
Tips
A common mistake would be to assume the water is in a compressed liquid state and use saturated liquid properties. However, the specific volume given is much larger than the specific volume of saturated liquid at that temperature. Therefore, we must consider the superheated vapor region.
AI-generated content may contain errors. Please verify critical information