At p = 28 MPa, T = 700°C, find v in m³/kg and h in kJ/kg.
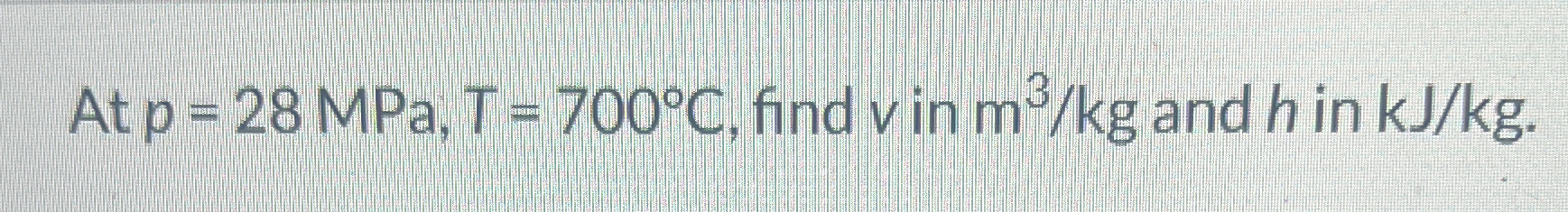
Understand the Problem
The problem requires determining specific volume (v) and specific enthalpy (h) of a substance at a given pressure (p) of 28 MPa and temperature (T) of 700°C. This typically involves using thermodynamic tables or equations of state for the particular substance to find these properties.
Answer
Values for specific volume (v) and specific enthalpy (h) at p = 28 MPa and T = 700°C can be found using steam tables or thermodynamic property software.
To determine the specific volume (v) and specific enthalpy (h) of water at p = 28 MPa and T = 700°C, you would typically use steam tables or thermodynamic property software. Since I don't have access to these tools, I am unable to provide the exact values. Consult a thermodynamics textbook or online resource with steam tables to find the values.
Answer for screen readers
To determine the specific volume (v) and specific enthalpy (h) of water at p = 28 MPa and T = 700°C, you would typically use steam tables or thermodynamic property software. Since I don't have access to these tools, I am unable to provide the exact values. Consult a thermodynamics textbook or online resource with steam tables to find the values.
More Information
Thermodynamic property tables (such as steam tables) are essential for finding properties of substances at various conditions. These tables typically list properties like specific volume, enthalpy, internal energy, and entropy for different temperatures and pressures.
Tips
A common mistake is to interpolate incorrectly between values in the steam tables. Always double-check your interpolation calculations.
Sources
AI-generated content may contain errors. Please verify critical information