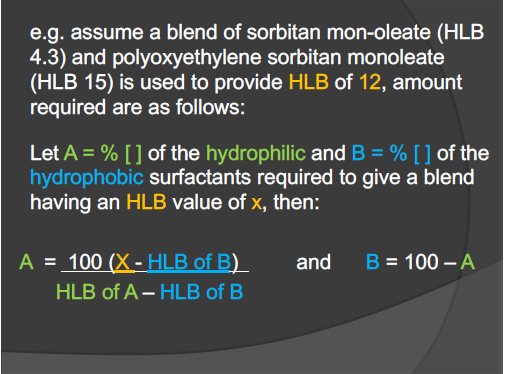
Assume a blend of sorbitan mon-oleate (HLB 4.3) and polyoxyethylene sorbitan monoleate (HLB 15) is used to provide an HLB of 12. Let A = % of the hydrophilic and B = % of the hydro... Assume a blend of sorbitan mon-oleate (HLB 4.3) and polyoxyethylene sorbitan monoleate (HLB 15) is used to provide an HLB of 12. Let A = % of the hydrophilic and B = % of the hydrophobic surfactants required to give a blend having an HLB value of x. Then A = 100 (X - HLB of B) / (HLB of A - HLB of B) and B = 100 - A.
Understand the Problem
The question is asking how to calculate the percentages of hydrophilic and hydrophobic surfactants required to achieve a specific HLB value based on the given formulas. It involves substituting the known HLB values into the equations for A and B.
Answer
$A \approx 28.03\%, B \approx 71.97\%$
Answer for screen readers
The required percentages of surfactants are approximately:
- $A \approx 28.03%$ (hydrophilic)
- $B \approx 71.97%$ (hydrophobic)
Steps to Solve
-
Identify Given Values
Given:
- HLB of A (sorbitan mon-oleate) = 4.3
- HLB of B (polyoxyethylene sorbitan monoleate) = 15
- Desired HLB value (X) = 12
-
Substitute Values into Formula for A
Use the formula: $$ A = 100 \times \frac{X - HLB_{B}}{HLB_{A} - HLB_{B}} $$ Substitute the known values: $$ A = 100 \times \frac{12 - 15}{4.3 - 15} $$
-
Calculate A
Simplify the calculation: $$ A = 100 \times \frac{-3}{-10.7} $$ Now, calculating: $$ A \approx 28.03 $$
-
Calculate B
Use the relationship: $$ B = 100 - A $$ Substitute the calculated value of A: $$ B = 100 - 28.03 $$ Calculate: $$ B \approx 71.97 $$
The required percentages of surfactants are approximately:
- $A \approx 28.03%$ (hydrophilic)
- $B \approx 71.97%$ (hydrophobic)
More Information
The Hydrophilic-Lipophilic Balance (HLB) system is crucial for formulating emulsions. The calculated percentages help in achieving an emulsion with a desired stability and consistency, ensuring effective dispersion in formulations.
Tips
- Misunderstanding HLB Concept: Ensure clarity on whether surfactants are more hydrophilic or hydrophobic.
- Incorrect Substitution: Double-check that the correct values for HLB of A, B, and desired HLB are used in calculations.
- Neglecting Signs in Calculations: Pay close attention to signs while performing arithmetic, especially in subtraction.
AI-generated content may contain errors. Please verify critical information