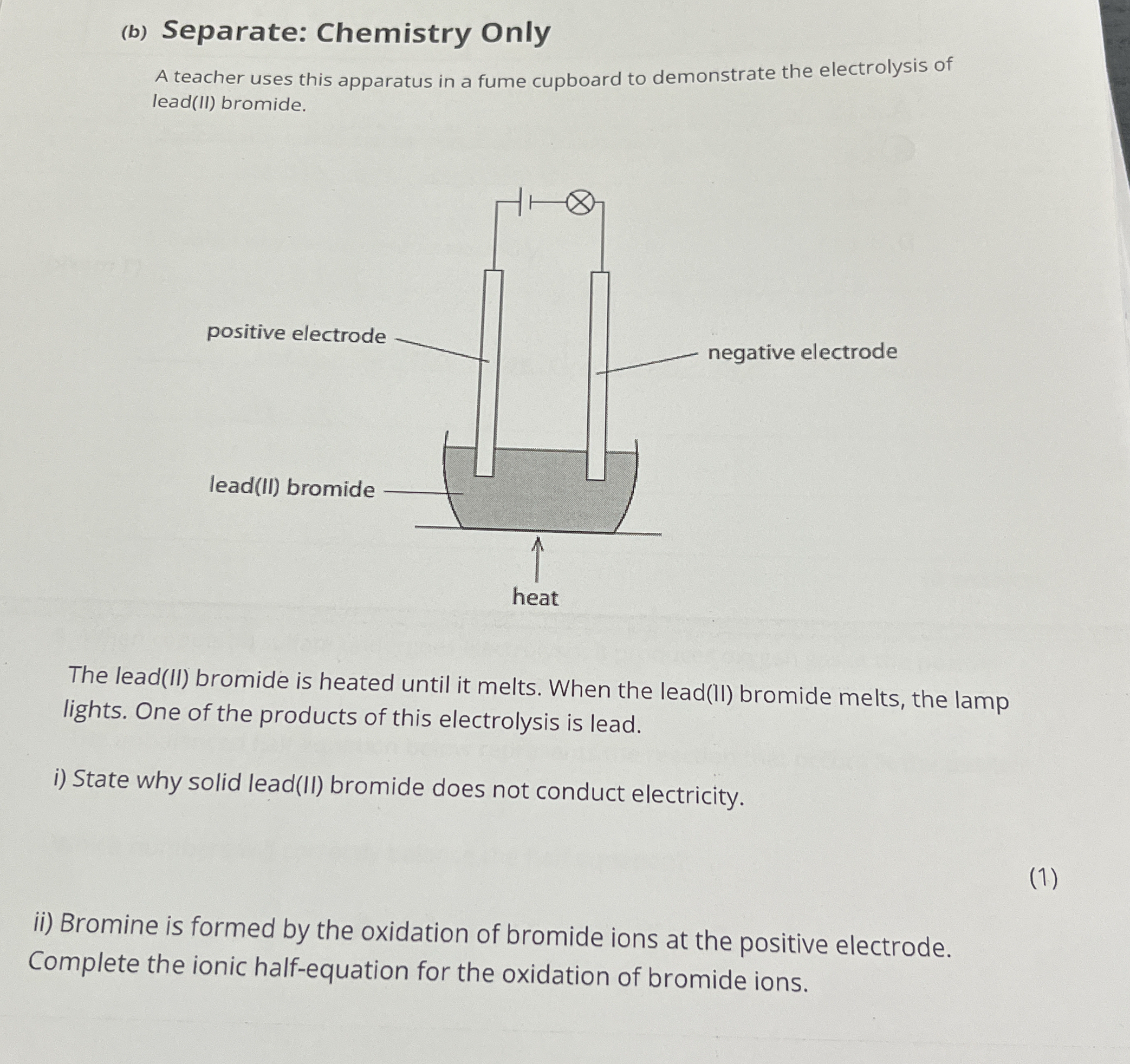
A teacher uses this apparatus in a fume cupboard to demonstrate the electrolysis of lead(II) bromide. The lead(II) bromide is heated until it melts. When the lead(II) bromide melts... A teacher uses this apparatus in a fume cupboard to demonstrate the electrolysis of lead(II) bromide. The lead(II) bromide is heated until it melts. When the lead(II) bromide melts, the lamp lights. One of the products of this electrolysis is lead. i) State why solid lead(II) bromide does not conduct electricity. ii) Bromine is formed by the oxidation of bromide ions at the positive electrode. Complete the ionic half-equation for the oxidation of bromide ions.
Understand the Problem
This question is about the electrolysis of lead(II) bromide. It asks why solid lead(II) bromide doesn't conduct electricity and requests completion of the ionic half-equation for the oxidation of bromide ions.
Answer
i) Ions are not free to move in solid lead(II) bromide. ii) 2Br- → Br2 + 2e-
i) Solid lead(II) bromide does not conduct electricity because the ions are not free to move, they are in a fixed position. ii) 2Br- → Br2 + 2e-
Answer for screen readers
i) Solid lead(II) bromide does not conduct electricity because the ions are not free to move, they are in a fixed position. ii) 2Br- → Br2 + 2e-
More Information
In solid form, ionic compounds like lead(II) bromide have their ions held in a fixed lattice structure, preventing them from moving and carrying a charge. When melted, the ions are free to move and conduct electricity.
Tips
Make sure to include state symbols in redox equations, this is a common mistake.
Sources
AI-generated content may contain errors. Please verify critical information