A series of multiple-choice chemistry questions:
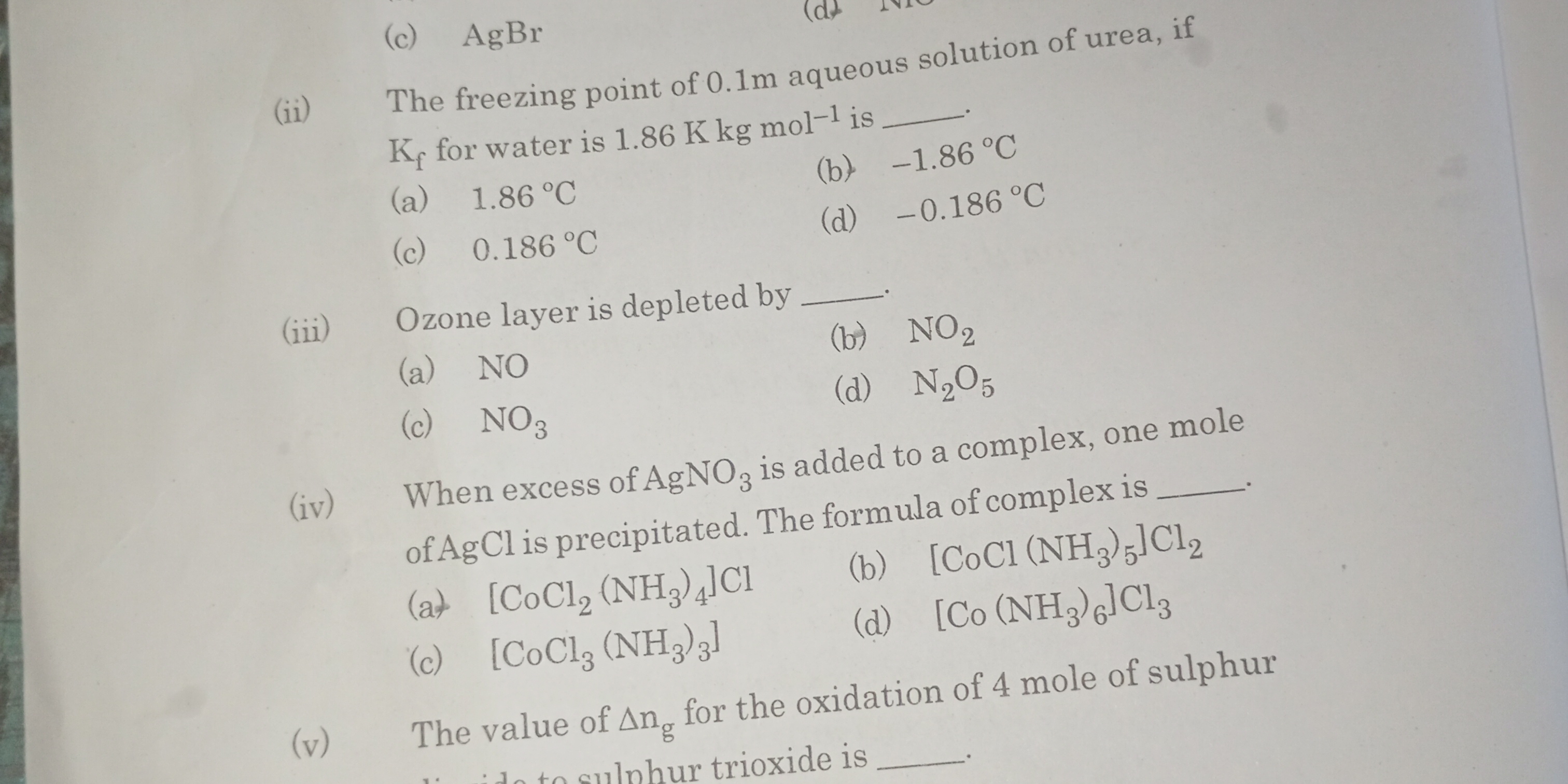
Understand the Problem
The image presents a series of multiple-choice chemistry questions. We need to classify the first four questions based on their subject matter and identify their category. The questions cover topics like freezing point depression, ozone depletion, complex compounds, and oxidation.
Answer
(ii) d, (iii) a, (iv) a
Here are the answers to the multiple-choice questions: (ii) (d) -0.186 °C (iii) (a) NO (iv) (a) [CoCl2 (NH3)4]Cl
Answer for screen readers
Here are the answers to the multiple-choice questions: (ii) (d) -0.186 °C (iii) (a) NO (iv) (a) [CoCl2 (NH3)4]Cl
More Information
Here's how to solve the questions: (ii) \Delta T_f = K_f * m = 1.86 K kg/mol * 0.1 mol/kg = 0.186 K. Therefore the freezing point is 0 - 0.186 = -0.186°C (iii) Ozone is depleted by NO (iv) Since one mole of AgCl is precipitated, it means one Cl- ion is outside the coordination sphere. Therefore the answer is [CoCl2(NH3)4]Cl
Tips
Pay attention to units and signs of the constants.
AI-generated content may contain errors. Please verify critical information