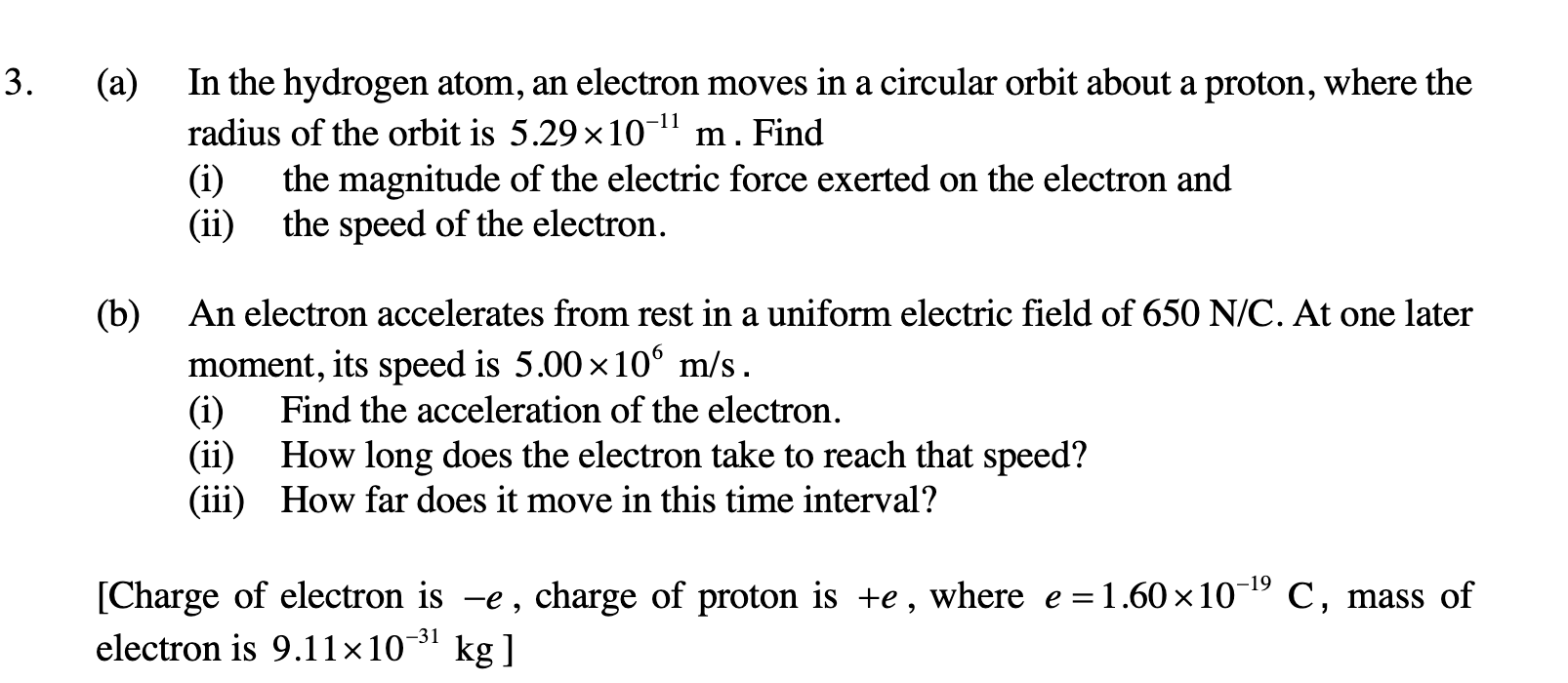
a) In the hydrogen atom, an electron moves in a circular orbit about a proton, where the radius of the orbit is 5.29 x 10^-11 m. Find: i) the magnitude of the electric force exerte... a) In the hydrogen atom, an electron moves in a circular orbit about a proton, where the radius of the orbit is 5.29 x 10^-11 m. Find: i) the magnitude of the electric force exerted on the electron and ii) the speed of the electron. b) An electron accelerates from rest in a uniform electric field of 650 N/C. At one later moment, its speed is 5.00 x 10^6 m/s. i) Find the acceleration of the electron. ii) How long does the electron take to reach that speed? iii) How far does it move in this time interval? [Charge of electron is -e, charge of proton is +e, where e = 1.60 x 10^-19 C, mass of electron is 9.11 x 10^-31 kg]
Understand the Problem
The question describes two scenarios involving electrons and asks for calculations related to their motion and the forces acting upon them. Part (a) concerns an electron orbiting a proton in a hydrogen atom, requiring the calculation of electric force and electron speed. Part (b) involves an electron accelerating in a uniform electric field, asking for the calculation of acceleration, time to reach a specific speed, and distance traveled during that time. We will use concepts from electromagnetism and kinematics to solve this problem.
Answer
(a) (i) $8.23 \times 10^{-8} \, \text{N}$, (ii) $2.19 \times 10^6 \, \text{m/s}$ (b) (i) $1.14 \times 10^{14} \, \text{m/s}^2$, (ii) $4.38 \times 10^{-8} \, \text{s}$, (iii) $0.0109 \, \text{m}$
Answer for screen readers
(a) (i) $8.23 \times 10^{-8} , \text{N}$ (ii) $2.19 \times 10^6 , \text{m/s}$ (b) (i) $1.14 \times 10^{14} , \text{m/s}^2$ (ii) $4.38 \times 10^{-8} , \text{s}$ (iii) $0.0109 , \text{m}$
Steps to Solve
- Calculate the electric force in part (a)(i)
The magnitude of the electric force between the electron and proton is given by Coulomb's law:
$F = k \frac{|q_1 q_2|}{r^2}$
where $k = 8.9875 \times 10^9 , \text{N m}^2/\text{C}^2$, $q_1 = q_p = +1.60 \times 10^{-19} , \text{C}$, $q_2 = q_e = -1.60 \times 10^{-19} , \text{C}$, and $r = 5.29 \times 10^{-11} , \text{m}$. Substituting these values:
$F = (8.9875 \times 10^9) \frac{(1.60 \times 10^{-19})^2}{(5.29 \times 10^{-11})^2}$
- Calculate the electron speed in part (a)(ii)
Since the electron moves in a circular orbit, the electric force provides the centripetal force:
$F = \frac{mv^2}{r}$
where $m = 9.11 \times 10^{-31} , \text{kg}$. Solving for $v$:
$v = \sqrt{\frac{Fr}{m}}$
- Calculate the acceleration of the electron in part (b)(i)
The electric force on the electron due to the electric field is $F = qE$, and using Newton's second law, $F = ma$, we can find the acceleration:
$a = \frac{qE}{m}$
where $E = 650 , \text{N/C}$, $q = -1.60 \times 10^{-19} , \text{C}$ and $m = 9.11 \times 10^{-31} , \text{kg}$.
- Calculate the time to reach the speed in part (b)(ii)
Using the kinematic equation $v = v_0 + at$, where $v_0 = 0 , \text{m/s}$ (starts from rest) and $v = 5.00 \times 10^6 , \text{m/s}$, we solve for $t$:
$t = \frac{v}{a}$
- Calculate the distance traveled in part (b)(iii)
Using the kinematic equation $x = v_0t + \frac{1}{2}at^2$ with $v_0 = 0$:
$x = \frac{1}{2}at^2$
(a) (i) $8.23 \times 10^{-8} , \text{N}$ (ii) $2.19 \times 10^6 , \text{m/s}$ (b) (i) $1.14 \times 10^{14} , \text{m/s}^2$ (ii) $4.38 \times 10^{-8} , \text{s}$ (iii) $0.0109 , \text{m}$
More Information
The electric force in the Hydrogen atom's electron is extremely small due to the electron's tiny charge and the very small radius of orbit, but it is enough to keep it in orbit! The electron's acceleration in the electric field is very large because of its small mass.
Tips
- Forgetting to square the radius in Coulomb's Law.
- Using the wrong sign for the electron charge, although the magnitude is what matters for force calculations in this context.
- Using incorrect units, especially when mixing meters, centimeters, or millimeters.
- Not converting all values to SI units before performing calculations.
- Confusing the mass of an electron with the mass of a proton.
AI-generated content may contain errors. Please verify critical information