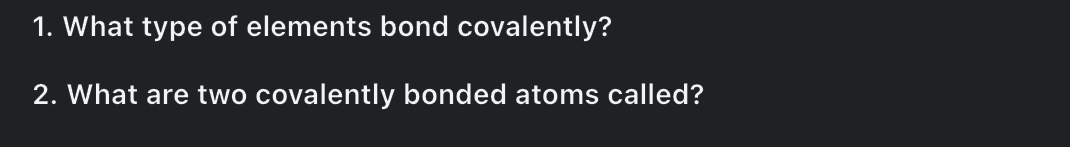
1. What type of elements bond covalently? 2. What are two covalently bonded atoms called?
Understand the Problem
The image presents two questions about covalent bonds in chemistry. The first asks about the type of elements that form covalent bonds. The second asks what two covalently bonded atoms are called.
Answer
1. Nonmetals. 2. Molecule.
- Nonmetals bond covalently through sharing electrons to achieve stability.
- Two covalently bonded atoms are called a molecule.
Answer for screen readers
- Nonmetals bond covalently through sharing electrons to achieve stability.
- Two covalently bonded atoms are called a molecule.
More Information
Covalent bonds are strong bonds that involve the sharing of electron pairs between atoms, typically nonmetals. These bonds allow atoms to achieve a stable electron configuration, similar to noble gases.
Tips
A common mistake is thinking that covalent bonds only occur between the same element. Covalent bonds can occur between different nonmetal elements.
Sources
- Covalent Bonds - Chemistry LibreTexts - chem.libretexts.org
- 9.2: Covalent Bond - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information