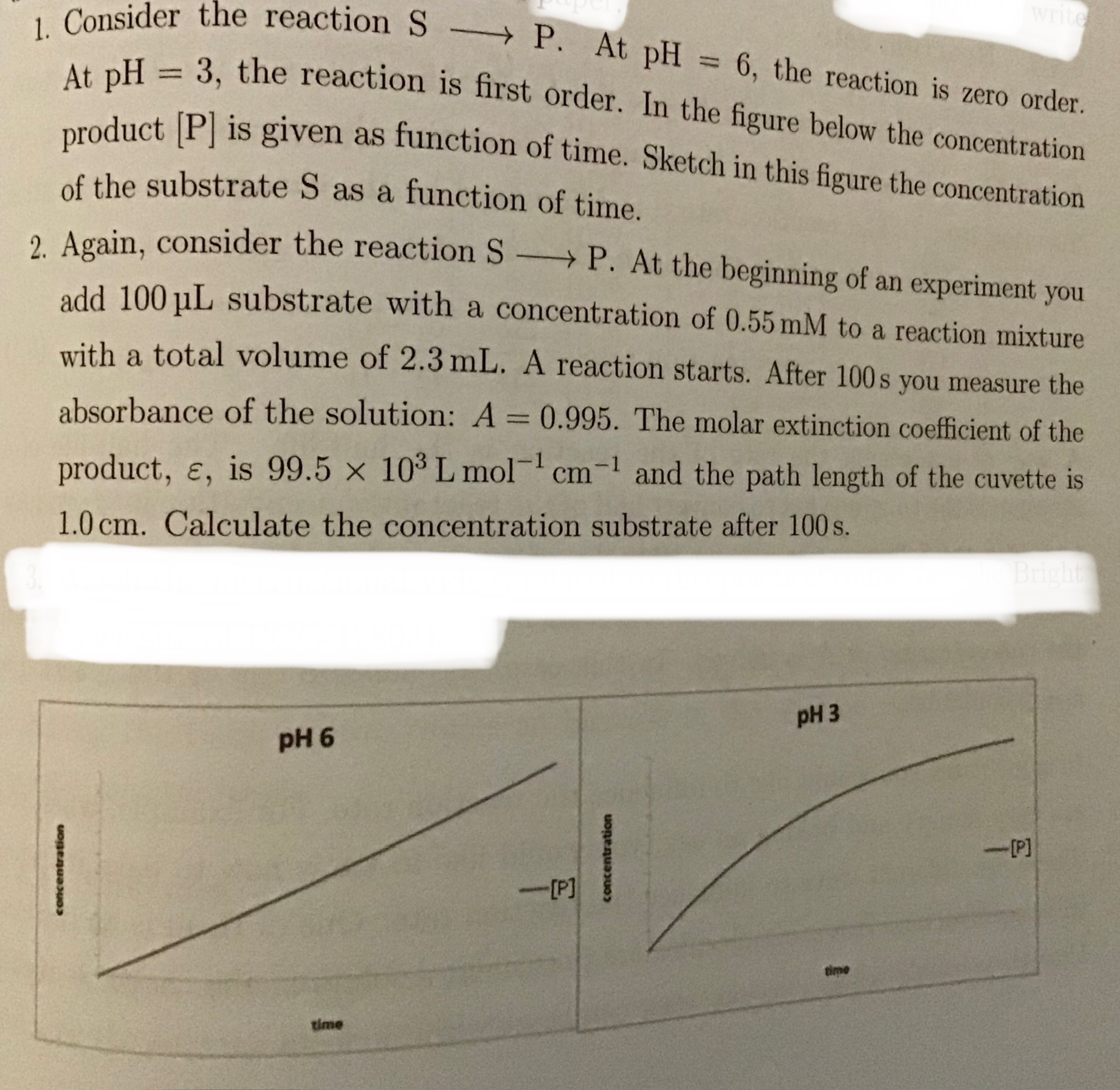
1. Consider the reaction S -> P. At pH = 3, the reaction is first order. At pH = 6, the reaction is zero order. In the figure below the concentration product [P] is given as functi... 1. Consider the reaction S -> P. At pH = 3, the reaction is first order. At pH = 6, the reaction is zero order. In the figure below the concentration product [P] is given as function of time. Sketch in this figure the concentration of the substrate S as a function of time. 2. Again, consider the reaction S -> P. At the beginning of an experiment you add 100 µL substrate with a concentration of 0.55 mM to a reaction mixture with a total volume of 2.3 mL. A reaction starts. After 100s you measure the absorbance of the solution: A = 0.995. The molar extinction coefficient of the product, ε, is 99.5 × 10^3 L mol^-1 cm^-1 and the path length of the cuvette is 1.0 cm. Calculate the concentration substrate after 100 s.
Understand the Problem
The image presents two chemistry questions. The first question requires sketching concentration vs. time graphs for a reaction at two different pH levels (3 and 6), considering the reaction order at each pH. The second question involves calculating the concentration of the substrate after 100 seconds, given initial conditions, absorbance, molar extinction coefficient, and path length. We'll need to use the Beer-Lambert Law to solve the second problem.
Answer
1. For pH=6 the graph is a line with a negative slope, for pH=3 graph is exponential decay 2. $[S]_{100} = 0.0139 \text{ mM}$
Answer for screen readers
- For pH = 6, the graph should be a straight line with a negative slope, starting at $[S]_0$ and decreasing linearly with time
- For pH = 3, the graph should show exponential decay, starting at $[S]_0$ and decreasing, but with the rate of decrease slowing down over time
- The concentration of the substrate after 100 s is $0.0139 \text{ mM}$
Steps to Solve
- Sketching the concentration of S at pH = 6
Since the reaction is zero order at pH = 6, the rate of the reaction is independent of the substrate concentration i.e. the substrate S decreases linearly with time, and the plot of [S] vs time will be a straight line with a negative slope.
- Sketching the concentration of S at pH = 3
Since the reaction is first order at pH = 3, the rate of the reaction is proportional to the substrate concentration. Thus the substrate S decreases exponentially with time and the plot of [S] vs time will show a decay that starts rapidly and slows down as the reaction progresses.
- Calculating the initial concentration of substrate
The initial concentration of the added substrate must be calculated taking into account the dilution. $C_1V_1 = C_2V_2$ where $C_1$ is the initial concentration of the substrate, $V_1$ is the initial volume of the substrate, $C_2$ is the final concentration of the substrate, and $V_2$ is the final volume of the solution. $$ (0.55 \text{ mM})(100 \text{ } \mu \text{L}) = C_2(2.3 \text{ mL}) $$ First we must convert $100 \text{ } \mu \text{L}$ to mL $100 \text{ } \mu \text{L} = 0.1 \text{ mL}$ $$ (0.55 \text{ mM})(0.1 \text{ mL}) = C_2(2.3 \text{ mL}) $$ $$ C_2 = \frac{(0.55 \text{ mM})(0.1 \text{ mL})}{(2.3 \text{ mL})} = 0.0239 \text{ mM} $$ The intial concentration of the substrate in the mixture is .0239 mM
- Calculate [P] after 100 seconds using Beer-Lambert Law
The Beer-Lambert Law is given by: $ A = \epsilon \cdot l \cdot c $
where $A$ is the absorbance, $\epsilon$ is the molar extinction coefficient, $l$ is the path length, and $c$ is the concentration. We can rearrange the equation to solve for the concentration of the product [P]:
$ c = \frac{A}{\epsilon \cdot l} $
Plugging in the values:
$ c = \frac{0.995}{(99.5 \times 10^3 \text{ L mol}^{-1} \text{cm}^{-1}) \cdot (1.0 \text{ cm})} $
$ c = \frac{0.995}{99.5 \times 10^3} \text{ mol L}^{-1} = 1.0 \times 10^{-5} \text{ mol L}^{-1} $ Which can be expressed as $ c = 0.01 \text{ mM} $ Therefore the concentration of product is 0.01 mM
- Calculating [S] after 100 seconds
Since $S \longrightarrow P$ at any time $t$, $[S]_0 = [S]_t + [P]_t$, thus $[S]_t = [S]_0 - [P]_t$
$[S]_{100} = 0.0239 \text{ mM} - 0.01 \text{ mM} = 0.0139 \text{ mM}$
- For pH = 6, the graph should be a straight line with a negative slope, starting at $[S]_0$ and decreasing linearly with time
- For pH = 3, the graph should show exponential decay, starting at $[S]_0$ and decreasing, but with the rate of decrease slowing down over time
- The concentration of the substrate after 100 s is $0.0139 \text{ mM}$
More Information
The Beer-Lambert law describes how the intensity of light is reduced as it passes through a substance. The law is commonly applied in chemistry for the analysis of various substances/reactions.
Tips
A common mistake is forgetting the dilution factor when calculating the initial substrate concentration, leading to incorrect concentrations of [S] at t=100s. Additionally, students may forget the units, or convert the units incorrectly, leading to the wrong answer.
AI-generated content may contain errors. Please verify critical information