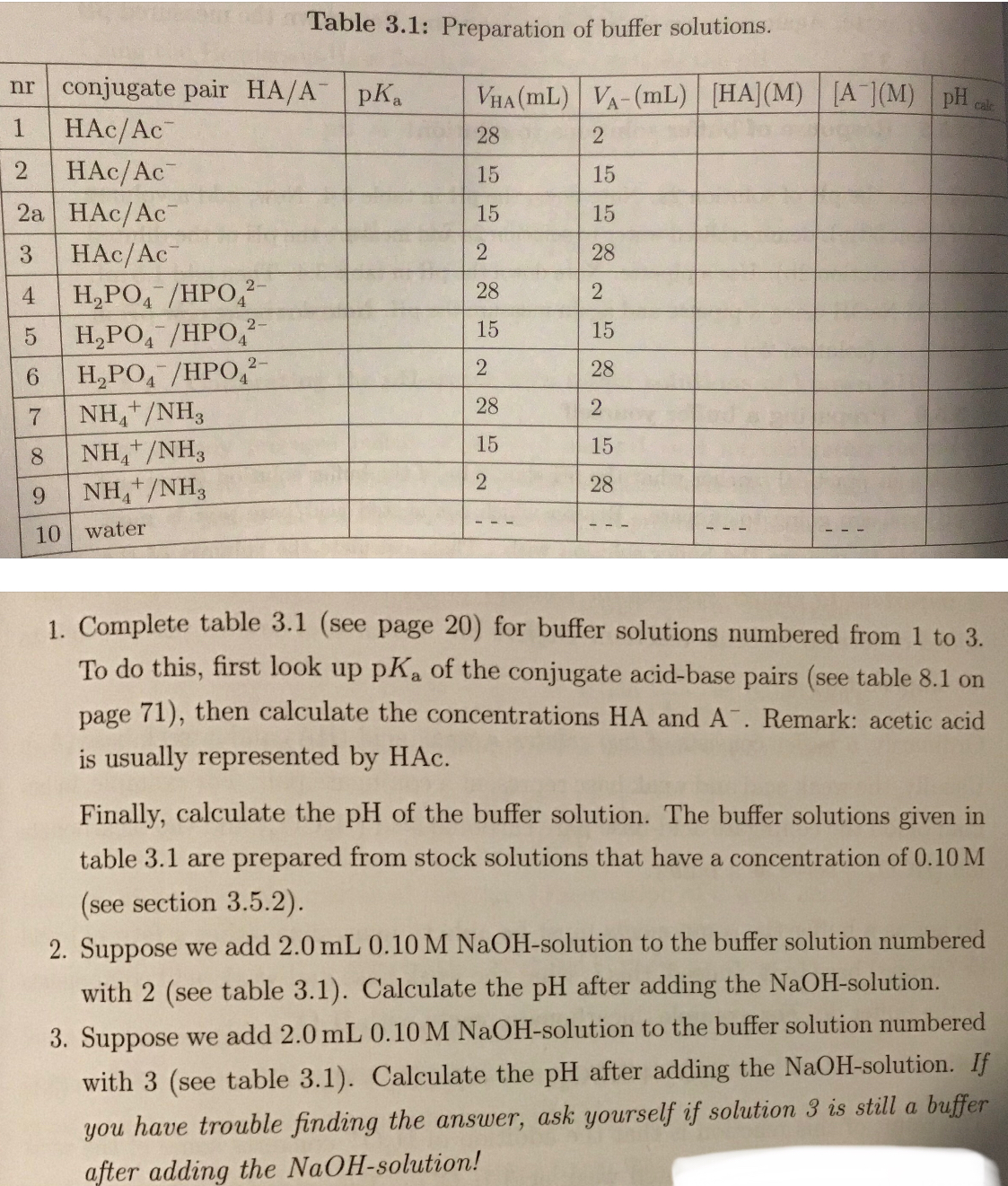
1. Complete table 3.1 (see page 20) for buffer solutions numbered from 1 to 3. To do this, first look up pKa of the conjugate acid-base pairs (see table 8.1 on page 71), then calcu... 1. Complete table 3.1 (see page 20) for buffer solutions numbered from 1 to 3. To do this, first look up pKa of the conjugate acid-base pairs (see table 8.1 on page 71), then calculate the concentrations HA and A-. Remark: acetic acid is usually represented by HAc. Finally, calculate the pH of the buffer solution. The buffer solutions given in table 3.1 are prepared from stock solutions that have a concentration of 0.10 M (see section 3.5.2). 2. Suppose we add 2.0 mL 0.10 M NaOH-solution to the buffer solution numbered with 2 (see table 3.1). Calculate the pH after adding the NaOH-solution. 3. Suppose we add 2.0 mL 0.10 M NaOH-solution to the buffer solution numbered with 3 (see table 3.1). Calculate the pH after adding the NaOH-solution. If you have trouble finding the answer, ask yourself if solution 3 is still a buffer after adding the NaOH-solution!
Understand the Problem
The image contains instructions to complete a table (Table 3.1) for buffer solutions, calculate the pH of solutions after adding NaOH, and additional context regarding the buffer solutions. Specifically:
- Complete Table 3.1: This requires finding the pKa values for the conjugate acid-base pairs, calculating the concentrations of HA and A-, and then calculating the pH for buffer solutions numbered 1 to 3.
- NaOH Addition: Calculate the pH after adding 2.0 mL of 0.10 M NaOH to buffer solutions numbered 2 and 3 in Table 3.1.
The task involves understanding buffer solutions, equilibrium, and pH calculations.
Answer
1. See completed table in "answer" 2. pH of solution 2 after adding NaOH: 4.88 3. pH of solution 3 after adding NaOH: 8.87
Answer for screen readers
- Completed Table 3.1 (Rows 1-3):
nr conjugate pair HA/A- pKa VHA(mL) VA-(mL) [HA] (M) [A-] (M) pH calc 1 HAc/Ac- 4.76 28 2 0.0933 0.00667 3.62 2 HAc/Ac- 4.76 15 15 0.05 0.05 4.76 3 HAc/Ac- 4.76 2 28 0.00667 0.0933 5.91 - pH after adding NaOH to buffer solution 2: 4.88
- pH after adding NaOH to buffer solution 3: 8.87
Steps to Solve
- Find the pKa value
The conjugate acid-base pair is HAc/Ac- (acetic acid/acetate). From standard tables (or table 8.1 on page 71 as mentioned), the $pK_a$ of acetic acid is 4.76.
- Calculate [HA] and [A-] for buffer solution 1
The total volume of the solution is $V_T = 28 \text{ mL} + 2 \text{ mL} = 30 \text{ mL}$. The concentration of HA (HAc) is calculated as follows: $$ [HA] = \frac{V_{HA} \times [\text{stock}]}{V_T} = \frac{28 \text{ mL} \times 0.10 \text{ M}}{30 \text{ mL}} = 0.0933 \text{ M} $$ The concentration of A- (Ac-) is calculated as follows: $$ [A^-] = \frac{V_{A^-} \times [\text{stock}]}{V_T} = \frac{2 \text{ mL} \times 0.10 \text{ M}}{30 \text{ mL}} = 0.00667 \text{ M} $$
- Calculate the pH of buffer solution 1 using the Henderson-Hasselbalch equation
The Henderson-Hasselbalch equation is: $$ pH = pK_a + \log_{10} \frac{[A^-]}{[HA]} $$ $$ pH = 4.76 + \log_{10} \frac{0.00667}{0.0933} = 4.76 + \log_{10}(0.0715) = 4.76 - 1.145 = 3.615 $$ Therefore, the pH of buffer solution 1 is approximately 3.62.
- Calculate [HA] and [A-] for buffer solution 2
The total volume of the solution is $V_T = 15 \text{ mL} + 15 \text{ mL} = 30 \text{ mL}$. The concentration of HA (HAc) is calculated as follows: $$ [HA] = \frac{V_{HA} \times [\text{stock}]}{V_T} = \frac{15 \text{ mL} \times 0.10 \text{ M}}{30 \text{ mL}} = 0.05 \text{ M} $$ The concentration of A- (Ac-) is calculated as follows: $$ [A^-] = \frac{V_{A^-} \times [\text{stock}]}{V_T} = \frac{15 \text{ mL} \times 0.10 \text{ M}}{30 \text{ mL}} = 0.05 \text{ M} $$
-
Calculate the pH of buffer solution 2 using the Henderson-Hasselbalch equation $$ pH = pK_a + \log_{10} \frac{[A^-]}{[HA]} $$ $$ pH = 4.76 + \log_{10} \frac{0.05}{0.05} = 4.76 + \log_{10}(1) = 4.76 + 0 = 4.76 $$ Therefore, the pH of buffer solution 2 is 4.76.
-
Calculate [HA] and [A-] for buffer solution 3
The total volume of the solution is $V_T = 2 \text{ mL} + 28 \text{ mL} = 30 \text{ mL}$.
The concentration of HA (HAc) is calculated as follows: $$ [HA] = \frac{V_{HA} \times [\text{stock}]}{V_T} = \frac{2 \text{ mL} \times 0.10 \text{ M}}{30 \text{ mL}} = 0.00667 \text{ M} $$ The concentration of A- (Ac-) is calculated as follows: $$ [A^-] = \frac{V_{A^-} \times [\text{stock}]}{V_T} = \frac{28 \text{ mL} \times 0.10 \text{ M}}{30 \text{ mL}} = 0.0933 \text{ M} $$
- Calculate the pH of buffer solution 3 using the Henderson-Hasselbalch equation
$$ pH = pK_a + \log_{10} \frac{[A^-]}{[HA]} $$ $$ pH = 4.76 + \log_{10} \frac{0.0933}{0.00667} = 4.76 + \log_{10}(13.99) = 4.76 + 1.146 = 5.906 $$ Therefore, the pH of buffer solution 3 is approximately 5.91.
- Calculate the new pH of buffer solution 2 after adding NaOH
- Determine the moles of NaOH added: $ \text{moles of NaOH} = 0.002 \text{ L} \times 0.10 \text{ M} = 0.0002 \text{ moles} $
- The NaOH will react with the acetic acid (HA) to form acetate (A-).
- New moles of HA: $ (0.05 \text{ M} \times 0.03 \text{ L}) - 0.0002 \text{ moles} = 0.0015 \text{ moles} - 0.0002 \text{ moles} = 0.0013 \text{ moles} $
- New moles of A-: $ (0.05 \text{ M} \times 0.03 \text{ L}) + 0.0002 \text{ moles} = 0.0015 \text{ moles} + 0.0002 \text{ moles} = 0.0017 \text{ moles} $
- New total volume: $30 \text{ mL} + 2 \text{ mL} = 32 \text{ mL} = 0.032 \text{ L}$
- New concentration of HA: $ [HA] = \frac{0.0013 \text{ moles}}{0.032 \text{ L}} = 0.0406 \text{ M} $
- New concentration of A-: $ [A^-] = \frac{0.0017 \text{ moles}}{0.032 \text{ L}} = 0.0531 \text{ M} $
- Calculate the new pH: $ pH = 4.76 + \log_{10} \frac{0.0531}{0.0406} = 4.76 + \log_{10}(1.308) = 4.76 + 0.117 = 4.877 $ Therefore, the pH of buffer solution 2 after adding NaOH is approximately 4.88.
- Calculate the new pH of buffer solution 3 after adding NaOH
- Determine the moles of NaOH added: $ \text{moles of NaOH} = 0.002 \text{ L} \times 0.10 \text{ M} = 0.0002 \text{ moles} $
- Calculate the initial moles of HA and A-$: $ \text{moles of HA} = 0.00667 \text{M} \times 0.03 \text{ L} = 0.0002 \text{ moles} $ $ \text{moles of A}^- = 0.0933 \text{ M} \times 0.03 \text{ L} = 0.0028 \text{ moles} $
- Since the moles of NaOH added (0.0002 moles) is equal to the moles of HA, the NaOH will completely react with all of the acetic acid (HA), converting it to acetate (A-).
- New moles of HA: $ 0.0002 \text{ moles} - 0.0002 \text{ moles} = 0 \text{ moles} $
- New moles of A-: $ 0.0028 \text{ moles} + 0.0002 \text{ moles} = 0.0030 \text{ moles} $
- New total volume: $30 \text{ mL} + 2 \text{ mL} = 32 \text{ mL} = 0.032 \text{ L}$
- New concentration of A-: $ [A^-] = \frac{0.003 \text{ moles}}{0.032 \text{ L}} = 0.09375 \text{ M} $
Since all of the HA has been converted to A-, we have a solution of just $A^-$. The $pH$ is now determined by the hydrolysis of the $A^-$. $$ A^- + H_2O \rightleftharpoons HA + OH^- $$ We need to calculate the $K_b$ for the acetate ion: $$ K_b = \frac{K_w}{K_a} = \frac{1.0 \times 10^{-14}}{1.74 \times 10^{-5}} = 5.747 \times 10^{-10} $$ Using the approximation that $ [OH^-] = \sqrt{K_b \times [A^-]} $: $$ [OH^-] = \sqrt{5.747 \times 10^{-10} \times 0.09375} = \sqrt{5.388 \times 10^{-11}} = 7.34 \times 10^{-6} \text{ M} $$ Now we calculate the $pOH$: $$ pOH = - \log_{10} [OH^-] = - \log_{10} (7.34 \times 10^{-6}) = 5.134 $$ And finally, we find the $pH$: $$ pH = 14 - pOH = 14 - 5.134 = 8.866 $$ Therefore, the pH of the solution 3 after the addition of NaOH is approximately 8.87.
- Completed Table 3.1 (Rows 1-3):
nr conjugate pair HA/A- pKa VHA(mL) VA-(mL) [HA] (M) [A-] (M) pH calc 1 HAc/Ac- 4.76 28 2 0.0933 0.00667 3.62 2 HAc/Ac- 4.76 15 15 0.05 0.05 4.76 3 HAc/Ac- 4.76 2 28 0.00667 0.0933 5.91 - pH after adding NaOH to buffer solution 2: 4.88
- pH after adding NaOH to buffer solution 3: 8.87
More Information
The Henderson-Hasselbalch equation is very useful for calculating the pH of buffer solutions. It relates the pH of a buffer solution to the $pK_a$ of the acid and the ratio of the concentrations of the acid and its conjugate base. It is only accurate when the concentrations of the acid and base are high enough (typically at least 10 times the $K_a$ value)
Tips
- Forgetting to account for the volume change when adding NaOH.
- Incorrectly calculating the new moles of HA and A- after adding NaOH.
- Not realizing that in the solution 3, the addition of NaOH completely reacts with the HA, and so, one then needs to consider the hydrolysis of $A^-$ to calculate the $pH$ of such solution. Using the Henderson-Hasselbalch equation when there's only $A^-$ present will lead to an incorrect answer.
- Using the $K_a$ instead of the $K_b$ when calculating the $pH$ of a solution containing only $A^-$.
AI-generated content may contain errors. Please verify critical information