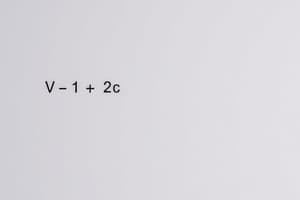Podcast
Questions and Answers
Under what conditions does a real gas behave most like an ideal gas?
Under what conditions does a real gas behave most like an ideal gas?
- High pressure and high temperature.
- High pressure and low temperature.
- Low pressure and low temperature.
- Low pressure and high temperature. (correct)
Which of the following is a key difference between real gases and ideal gases?
Which of the following is a key difference between real gases and ideal gases?
- Ideal gases experience significant intermolecular forces, while real gases do not.
- Molecules of ideal gases have a finite size, while real gases do not.
- Real gases strictly obey the classical gas laws under all conditions, while ideal gases do not.
- Real gases deviate from ideal behavior under high pressure and low temperature, while ideal gases do not. (correct)
According to the ideal gas law, if the number of moles and the temperature of a gas are kept constant, what will happen to the pressure if the volume is doubled?
According to the ideal gas law, if the number of moles and the temperature of a gas are kept constant, what will happen to the pressure if the volume is doubled?
- The pressure will double.
- The pressure will remain constant.
- The pressure will quadruple.
- The pressure will be halved. (correct)
Why do real gases deviate from ideal behavior at high pressures?
Why do real gases deviate from ideal behavior at high pressures?
Which of the following statements about real gases is correct?
Which of the following statements about real gases is correct?
Under what conditions does Charles Gay-Lussac's Law most accurately predict the behavior of real gases?
Under what conditions does Charles Gay-Lussac's Law most accurately predict the behavior of real gases?
How does increasing the pressure on a real gas affect its deviation from Charles Gay-Lussac's Law?
How does increasing the pressure on a real gas affect its deviation from Charles Gay-Lussac's Law?
What does a compressibility factor (Z) of less than 1 indicate about a gas?
What does a compressibility factor (Z) of less than 1 indicate about a gas?
Why is the coefficient of volume expansion not constant for all real gases at higher pressures, contrary to what Charles Gay-Lussac’s law suggests?
Why is the coefficient of volume expansion not constant for all real gases at higher pressures, contrary to what Charles Gay-Lussac’s law suggests?
A gas is found to have a compressibility factor $Z = 1.2$ at a certain temperature and pressure. What does this indicate about the molar volume ($V_m$) of the gas compared to the molar volume of an ideal gas ($V_{ideal}$) under the same conditions?
A gas is found to have a compressibility factor $Z = 1.2$ at a certain temperature and pressure. What does this indicate about the molar volume ($V_m$) of the gas compared to the molar volume of an ideal gas ($V_{ideal}$) under the same conditions?
Flashcards
Compressibility Factor (Z)
Compressibility Factor (Z)
A factor (Z) that quantifies the deviation of a real gas from ideal gas behavior. Z = PV/nRT
Charles Gay-Lussac's Law
Charles Gay-Lussac's Law
At constant pressure, the volume of a gas is directly proportional to its absolute temperature.
Coefficient of Volume Expansion
Coefficient of Volume Expansion
The fractional increase in volume per degree Celsius rise in temperature, approximately 1/273 per degree Celsius.
Deviation from Ideal Gas Law
Deviation from Ideal Gas Law
Signup and view all the flashcards
Causes of Real Gas Deviation
Causes of Real Gas Deviation
Signup and view all the flashcards
Ideal Gas
Ideal Gas
Signup and view all the flashcards
Ideal Gas Equation
Ideal Gas Equation
Signup and view all the flashcards
Real Gas
Real Gas
Signup and view all the flashcards
Intermolecular Forces Impact
Intermolecular Forces Impact
Signup and view all the flashcards
Deviation Conditions
Deviation Conditions
Signup and view all the flashcards
Study Notes
- An ideal gas, or perfect gas, is a hypothetical concept used in chemistry and physics to simplify the study of gases
- An ideal gas strictly obeys Boyle's Law (P∝1/V at constant T), Charles's Law (V∝T at constant P), and other classical gas laws under all conditions
Ideal Gas Equation
- PV=nRT, where:
- P = Pressure of the gas
- V = Volume of the gas
- n = Number of moles of the gas
- R = Universal gas constant
- T = Temperature in Kelvin
- Real gases follow gas laws only approximately under certain conditions and deviate from ideal gas behavior, especially with changes in P and T
- Real gases obey gas laws reasonably well when pressure is low or temperature is high, minimizing intermolecular forces effects
Intermolecular forces of real gases
- Under conditions, intermolecular forces and finite size of gas particles are minimal in real gases, behaving similarly to an ideal gas
- No perfect or ideal gases exist in nature; All gases such as O2, N2, and CO2 are real gases
- As pressure increases, molecules of a real gas are forced closer together, leading to increased impact of intermolecular forces and deviation from gas law predictions
- As T decreases, the kinetic energy of gas decreases, intermolecular forces become more significant, causing further deviations from ideal behavior
- All gases are real, and their behavior depends on pressure and temperature conditions
- Models like the Van der Waals equation are used to predict gas behavior under various conditions
Deviation from ideal behavior
- According to Boyle's law, PV is constant when T is unchanged
- Plotting PV against P should yield a horizontal line, however real gases do not follow this exactly
- Instead of a straight line, two different curves are obtained due to intermolecular forces and the finite size of gas molecules
- At low pressure, gases approximate ideal behavior, but deviations become more pronounced as pressure increases or temperature decreases
- For gases like CO and CH4, PV first decreases then increases with pressure
- For gases like hydrogen and helium, PV continuously increases with pressure
Compressibility factor
- Temperature and pressure effects can be studied in terms of compressibility factor 'z', where z = 1 for an ideal gas at all temperatures and pressures
- For real gases, z varies with changes in temperature and pressure
- Plots of z vs. P at different constant temperatures for nitrogen gas show varying curves
- As temperature increases the minimums in the curves shift upwards until z nears 1 over an appreciable pressure range
- The Boyle temperature or Boyle point is the temperature at which a real gas behaves like an ideal gas over an appreciable pressure range
- Above the Boyle temperature, a gas shows positive deviations only
Boyle's Law Deviations
- These deviations can be observed using the compressibility factor (Z), where: Z = PV/nRT
Charles Gay-Lussac's Law
- The volume of a gas is directly proportional to its absolute temperature when the P is kept constant → V/T=CONSTANT
- V1/T1 = V2/T2
Volume expansion
- All gases should have the same coefficient of volume expansion, defined as the fractional increase in volume per degree Celsius rise, and is temperature dependent
- For every 1°C rise in temperature, the volume of a gas should increase by 1/127 of its volume at 0°C
- This relationship holds only at low P, while at high P, gases deviate significantly from Charles Gay-Lussac's law
Van der Waals Equation
- Understanding differences in gases helps refine gas laws for real-world applications, where corrections like the Van der Waals equation are used
- At low pressures, gas molecules are far apart, and intermolecular forces are minimal and gases follow Charles Gay-Lussac's law closely
- As pressure increases, molecules are forced closer, the gas may expand less than expected due to the intermolecular forces opposing the volume increase
- Charles Gay-Lussac's law is foundational for understanding the relationship between temperature and volume, but its assumptions are only valid at low pressures
Causes for Deviation of Real Gases
- Deviations occur because real gases do not behave ideally due to intermolecular forces and molecular size, which become more prominent as P increases
- Gas laws are derived from kinetic theory of gases based upon certain assumptions
- At high P or low T, two assumptions of kinetic theory of gases become invalid
Assumptions leading to deviation
- The volume occupied by gas molecules is negligible compared to the total volume of the gas
- The forces of attraction or repulsion between gas molecules are negligible
Conditions for valid assumptionss
- Assumptions are only valid if the pressure is low or the temp is high, so the distance between molecules is large
- If the pressure is high or the temperature is low, gas molecules get closer, invalidating these assumptions
- The forces of attraction or repulsion between the molecules are not negligible
Van der Waals Equation
- Johannes Diderik van der Waals made a breakthrough in understanding real gases in 1873
- The ideal gas law (PV=nRT) failed to describe gases accurately under high P and low T
- Real gases deviate from ideal behavior due to key factors: Intermolecular Forces and Finite Molecular Volume
Intermolecular forces
- Unlike ideal gases, real gas molecules attract each other, reducing the pressure exerted on container walls
Finite Molecular Volume
- Unlike point-like particles assumed in ideal gases, real gas molecules occupy space, reducing available volume for movement
Correction of Volume and Pressure
- Van der Waals obtained an equation for real gases by correcting for volume and pressure
- U is the actual volume occupied by one mole of gas molecules
- The effective volume occupied by gas molecules is actually four times the actual volume i.e., equal to 4 U
- Let it be represented by b. The volume b = co-volume or excluded volume.
- The free space for movement: (V- b).
- The gas molecule is attracted by all other gas molecules surrounding it
- For a molecule near the wall of the container, the molecules inside the gas exert some net inward pull on it
- The observed pressure is smaller than it would if there were no inward pull because it's "dragging back"
- Correction depends on: number of molecules surrounding a molecule, and the total number of molecules
- Each factor depends upon the density (ρ) of the gas
Van der Waals Equation
- The constants a and b are called van der Waals constants, and depend on gas nature, and independent of temperature and P
- For n moles of gas, the corrected pressure become P+an²/ V2 and the effective volume = V- nb
- Van der Waals equation takes the form: (P+an²/ V2) (V-nb) =nRT
Significance of Constants
- Values of "a" are greater for easily liquefiable gases than for so-called permanent gases
- The value of "a" increases with the ease of liquefaction, thus it measures intermolecular forces of attraction in a gas
- "b" is the "effective volume" of the gas molecules
- The constancy in the value of b confirms that gas molecules are incompressible across a range of temperaturs and pressures
Behavior of Real Gases
- At extremely low pressure V is very large
- The van der Waals equation reduces to PV = RT
- Gases obey the ideal gas equation
Moderate Pressure conditions
- As P increases, V decreases and a/V² increases
- The van der Waals equation reduces to PV = RT - a/V
- PV is less than RT by a factor of a/V
- As P increases, V decreases, so a/V increases
- PV decreases until a dip is obtained
High Pressure conditions
- V is so small such that b can no longer be neglected
- The van der Waals equation reduces to PV = RT + Pb
- PV is greater than RT by a factor of Pb
- As P is increased, the factor Pb increases, the product PV increases.
- At any P, if T is very high, the van der Waals equation reduces to PV = RT & the real gas behaves like an ideal gas
Exceptional Behavior of H and He
- For gases like hydrogen and helium, intermolecular forces of attraction are extremely small, so the van der Waals equation remains applicable
- The van der Waals equation is: P (V -b) = RT or PV = RT + Pb
- Hydrogen and helium show positive deviation only as the value of P increases
Kammerlingh-Onnes Virial Equation of State
- This is a thermodynamic model used to describe real gases, with a series expansion, and introduces a compressibility factor (Z) to calculate the power series
- PV= A + BP + CP2 + DP3 + or PV = RT ( 1 + BP + CP2 + DP3 + ...)
- The coefficients A, B, C etc., are first, second, third, etc., termed virial coefficients
- Points to note about the above equation:
- The virial coefficients are different for different gases
- At very low pressure, only the first virial coefficient is significant and equal to RT, i.e. A= RT
- At higher pressures, other virial coefficients become more important
- For any gas, values of A, B, C, are constant at constant temperature, but change with temperature
- The first virial coefficient A is always positive and increases with temperature
- Boyle's temperature is the temperature at which a real gas most acts like an ideal gas over a range of pressures
- At the Boyle's temperature, the compressibility factor (Z for real gases) = 1 for a range of pressures, which means the effects of intermolecular forces is minimized
Equations of State
- Besides van der Waals equations and the virial equation, there exist a number of equations to study P-V-T relations
- One such equation is the Berthelot Equation, which is an empirical modification of the van der Waals equation of state
Dieterici Equation
- This is an empirical model similar to the van der Waals equation, incorporating intermolecular forces
- Satisfactory results at moderate pressure
- At higher pressures, the results has great agreement with experimental data
- The Redlich-Kwong is an improved model for describing real gases, refining the Van der Waals equation for better accuracy at higher temperatures and pressures
- The Clausius Equation studies the variance a with temperature, Clausius
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.