Podcast
Questions and Answers
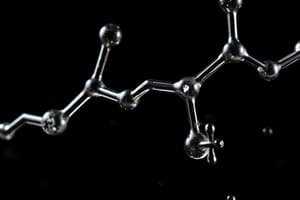
What is the functional group of the given compound?
What is the functional group of the given compound?
- Alkene
- Alkane
- Alkyne (correct)
- Aromatic
What type of bond is present between the two carbon atoms in the given compound?
What type of bond is present between the two carbon atoms in the given compound?
- Single bond
- Double bond
- Triple bond (correct)
- Quadruple bond
What is the general formula for an alkyne?
What is the general formula for an alkyne?
- CnH2n
- CnH2n-2 (correct)
- CnH2n+1
- CnH2n+2
What is the suffix used to name alkynes?
What is the suffix used to name alkynes?
How many pi bonds are present in the given compound?
How many pi bonds are present in the given compound?
What type of bond is holding the hydrogen in acidic compounds?
What type of bond is holding the hydrogen in acidic compounds?
What is the charge of the hydrogen ion when it separates from the acidic compound?
What is the charge of the hydrogen ion when it separates from the acidic compound?
What type of atoms are commonly found in acidic compounds?
What type of atoms are commonly found in acidic compounds?
What is the reason for the acidity of a compound?
What is the reason for the acidity of a compound?
What happens to the hydrogen ion when it separates from the acidic compound?
What happens to the hydrogen ion when it separates from the acidic compound?
What type of bond is present in acetylene or any alkyne with the triple bond at the end of the chain?
What type of bond is present in acetylene or any alkyne with the triple bond at the end of the chain?
What is the property exhibited by hydrogen attached to triply-bonded carbon?
What is the property exhibited by hydrogen attached to triply-bonded carbon?
What is the general formula of an alkyne with the triple bond at the end of the chain?
What is the general formula of an alkyne with the triple bond at the end of the chain?
What is the reason for the acidity of hydrogen attached to triply-bonded carbon?
What is the reason for the acidity of hydrogen attached to triply-bonded carbon?
What is acidity a measure of?
What is acidity a measure of?
What type of compound is acetylene or any alkyne with the triple bond at the end of the chain?
What type of compound is acetylene or any alkyne with the triple bond at the end of the chain?
What is the result of a compound losing a hydrogen ion?
What is the result of a compound losing a hydrogen ion?
What is the relationship between acidity and hydrogen ions?
What is the relationship between acidity and hydrogen ions?
What does a high acidity measure indicate?
What does a high acidity measure indicate?
What is the role of hydrogen ions in acidity?
What is the role of hydrogen ions in acidity?
What is the product formed when pent-1-ene is treated with bromine under conditions that facilitate the formation of a dihalide?
What is the product formed when pent-1-ene is treated with bromine under conditions that facilitate the formation of a dihalide?
What is the major product formed when excess bromine is added to pent-1-ene?
What is the major product formed when excess bromine is added to pent-1-ene?
What is the product formed when 1,3-butadiene is treated with bromine under similar conditions?
What is the product formed when 1,3-butadiene is treated with bromine under similar conditions?
Why does the treatment of 1,3-butadiene with bromine yield two products?
Why does the treatment of 1,3-butadiene with bromine yield two products?
What is the expected product when 1,3-butadiene is treated with bromine under similar conditions?
What is the expected product when 1,3-butadiene is treated with bromine under similar conditions?
Flashcards are hidden until you start studying
Study Notes
Acidity of Alkynes
- Acidity is the tendency of a compound to lose a hydrogen ion.
- Hydrogen attached to triply-bonded carbon (e.g., acetylene or RC=C-H) shows appreciable acidity due to the polar bond holding the hydrogen.
Reactions of Alkynes with Bromine
- 1-Butyne reacts with bromine to form 1,4-dibromo-2-butene.
- Further addition of bromine yields 1,2,4,5-tetrabromo pentane.
Reactions of Dienes with Bromine
- 1,3-Butadiene reacts with bromine to form 3,4-dibromo-1-butene and 1,4-dibromo-2-butene under similar conditions.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.