Podcast
Questions and Answers
What is the charge of the lithium ion when it loses an electron?
What is the charge of the lithium ion when it loses an electron?
- Li<sup>2+</sup>
- Li<sup>3+</sup>
- Li<sup>-</sup>
- Li<sup>+</sup> (correct)
Which of the following ions has the highest positive charge?
Which of the following ions has the highest positive charge?
- B<sup>3+</sup> (correct)
- Be<sup>2+</sup>
- Mg<sup>2+</sup>
- Na<sup>+</sup>
What is the Lewis dot representation of sodium before it forms an ion?
What is the Lewis dot representation of sodium before it forms an ion?
- Na<sup>3.</sup>
- Na<sup>2.</sup>
- Na<sup>.</sup> (correct)
- Na<sup>+</sup>
What is the electron configuration of magnesium after it has lost two electrons?
What is the electron configuration of magnesium after it has lost two electrons?
Which element listed has a neutral atom configuration of p+ and n° as p+ 5 n° 6?
Which element listed has a neutral atom configuration of p+ and n° as p+ 5 n° 6?
What characterizes a compound?
What characterizes a compound?
What occurs when a metal and a nonmetal bond together?
What occurs when a metal and a nonmetal bond together?
How many valence electrons does a neutral oxygen atom have?
How many valence electrons does a neutral oxygen atom have?
What happens to a metal when it forms an ionic bond?
What happens to a metal when it forms an ionic bond?
What is the overall charge of an oxygen atom that gains 2 electrons?
What is the overall charge of an oxygen atom that gains 2 electrons?
Flashcards
Atomic Number
Atomic Number
The number of protons in an atom, determining its atomic number and chemical identity.
Ionic Charge
Ionic Charge
The number of electrons an atom gains or loses to achieve a stable octet electron configuration.
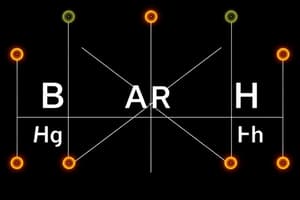
Ion B-R Diagram
Ion B-R Diagram
A diagram representing the electronic configuration of an ion, showing the distribution of electrons in shells and subshells.
Group 1 Elements: Ionic Charge
Group 1 Elements: Ionic Charge
Signup and view all the flashcards
Group 2 Elements: Ionic Charge
Group 2 Elements: Ionic Charge
Signup and view all the flashcards
Why do atoms bond?
Why do atoms bond?
Signup and view all the flashcards
Covalent bonding
Covalent bonding
Signup and view all the flashcards
What are valence electrons?
What are valence electrons?
Signup and view all the flashcards
Ionic bonding: Metals
Ionic bonding: Metals
Signup and view all the flashcards
Ionic bonding: Nonmetals
Ionic bonding: Nonmetals
Signup and view all the flashcards
Study Notes
Ion & Bohr-Rutherford Diagrams
-
Lithium (Li):
- Atomic structure shows 3 protons and 3 electrons.
- Forms a 1+ ion by losing 1 electron, resulting in 3 protons and 2 electrons.
- Lewis dot diagram shows 1 electron dot around the symbol.
-
Sodium (Na):
- Atomic structure shows 11 protons and 11 electrons.
- Forms a 1+ ion by losing 1 electron, resulting in 11 protons and 10 electrons.
- Lewis dot diagram shows a single electron dot around symbol.
-
Beryllium (Be):
- Atomic structure shows 4 protons and 4 electrons.
- Forms a 2+ ion by losing 2 electrons, resulting in 4 protons and 2 electrons.
- Lewis dot diagram shows 2 electron dots around the symbol.
-
Magnesium (Mg):
- Atomic structure shows 12 protons and 12 electrons.
- Forms a 2+ ion by losing 2 electrons, resulting in 12 protons and 10 electrons.
- Lewis dot diagram shows 2 electron dots around symbol.
-
Boron (B):
- Atomic structure shows 5 protons and 5 electrons.
- Forms a 3+ ion by losing 3 electrons, resulting in 5 protons and 2 electrons.
- Lewis dot diagram shows 3 electron dots around the symbol.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.