Podcast
Questions and Answers
What does the Z (trans) geometry of tamoxifen allow it to do?
What does the Z (trans) geometry of tamoxifen allow it to do?
- Enhance signaling for cell reproduction
- Alter the structure of other drugs
- Facilitate cell growth
- Bind tightly to the estrogen receptor (correct)
How do Z and E designations aid in naming alkenes?
How do Z and E designations aid in naming alkenes?
- By denoting molecular weight
- By distinguishing them based on theoretical symmetry
- By indicating stereoisomerism clearly (correct)
- By maximizing drug potency
Which statement about stereoisomerism is true?
Which statement about stereoisomerism is true?
- Stereoisomerism results from symmetrical arrangements
- Stereoisomers usually arise from asymmetry (correct)
- Stereoisomerism is irrelevant to the pharmaceutical industry
- Stereoisomers can easily be interconverted
What characterizes enantiomers?
What characterizes enantiomers?
What defines a racemic mixture?
What defines a racemic mixture?
Which term is synonymous with stereoisomerism?
Which term is synonymous with stereoisomerism?
Why is alkene geometry considered vital for pharmaceuticals?
Why is alkene geometry considered vital for pharmaceuticals?
What is indicated by the term 'optically inactive'?
What is indicated by the term 'optically inactive'?
What is the first step in determining the stereochemistry of a chiral carbon?
What is the first step in determining the stereochemistry of a chiral carbon?
When prioritizing atoms attached to a chiral carbon, which criterion should be used?
When prioritizing atoms attached to a chiral carbon, which criterion should be used?
How do you determine the priority order when two atoms attached to the chiral carbon are the same?
How do you determine the priority order when two atoms attached to the chiral carbon are the same?
In determining stereochemistry, if the priorities are arranged 1 → 2 → 3 in a clockwise direction, what classification is assigned?
In determining stereochemistry, if the priorities are arranged 1 → 2 → 3 in a clockwise direction, what classification is assigned?
Which option correctly identifies the order of atomic priorities for the chiral center in acebutolol?
Which option correctly identifies the order of atomic priorities for the chiral center in acebutolol?
What happens if the priority 4 atom is positioned at the front of the chiral carbon instead of at the back?
What happens if the priority 4 atom is positioned at the front of the chiral carbon instead of at the back?
Which of the following is true about the active enantiomer of acebutolol?
Which of the following is true about the active enantiomer of acebutolol?
When the chiral carbon has a hydrogen atom as priority 4, how is this atom oriented in the 3D representation?
When the chiral carbon has a hydrogen atom as priority 4, how is this atom oriented in the 3D representation?
Which statement best describes the chirality of a molecule with the priority 4 atom in the plane?
Which statement best describes the chirality of a molecule with the priority 4 atom in the plane?
What do enantiomers do to plane-polarized light?
What do enantiomers do to plane-polarized light?
What does a positive specific rotation indicate?
What does a positive specific rotation indicate?
What does the specific rotation of a compound tell you?
What does the specific rotation of a compound tell you?
Which of the following methods is used to assess chirality confidently?
Which of the following methods is used to assess chirality confidently?
What is the significance of the 3-point model of binding in the context of chiral interactions?
What is the significance of the 3-point model of binding in the context of chiral interactions?
In the context of enantiomers, what does a negative specific rotation signify?
In the context of enantiomers, what does a negative specific rotation signify?
Which of the following statements about chirality is incorrect?
Which of the following statements about chirality is incorrect?
What is required for an amine to be able to invert to opposite stereochemistry?
What is required for an amine to be able to invert to opposite stereochemistry?
When does an amine adopt partial double bond character?
When does an amine adopt partial double bond character?
Which statement about enantiomers is true?
Which statement about enantiomers is true?
What do Cahn-Ingold-Prelog rules determine?
What do Cahn-Ingold-Prelog rules determine?
Which of the following statements regarding inactive enantiomers is correct?
Which of the following statements regarding inactive enantiomers is correct?
What is the primary reason for the non-superimposable nature of enantiomers?
What is the primary reason for the non-superimposable nature of enantiomers?
What is the main application of Acamprosate?
What is the main application of Acamprosate?
Which of the following compounds is a veterinary mucolytic agent?
Which of the following compounds is a veterinary mucolytic agent?
Which enantiomer of Vigabatrin is active as an anti-convulsant?
Which enantiomer of Vigabatrin is active as an anti-convulsant?
What is the primary reason for marketing Fluoxetine as a racemate?
What is the primary reason for marketing Fluoxetine as a racemate?
How does the (S)-enantiomer of Warfarin compare to the (R)-enantiomer in terms of activity?
How does the (S)-enantiomer of Warfarin compare to the (R)-enantiomer in terms of activity?
Which statement is true regarding the activities of enantiomers?
Which statement is true regarding the activities of enantiomers?
What is the mechanism of action of the (S)-enantiomer of Vigabatrin?
What is the mechanism of action of the (S)-enantiomer of Vigabatrin?
In the context of Fluoxetine, what does the (R)-enantiomer's rapid elimination imply?
In the context of Fluoxetine, what does the (R)-enantiomer's rapid elimination imply?
Which of the following describes the actions of the (R)-enantiomer of Warfarin?
Which of the following describes the actions of the (R)-enantiomer of Warfarin?
What differentiates the activities of (R) and (S) enantiomers in many cases?
What differentiates the activities of (R) and (S) enantiomers in many cases?
Which of the following best describes the marketing of drugs as racemates?
Which of the following best describes the marketing of drugs as racemates?
What is a characteristic of a chiral carbon atom?
What is a characteristic of a chiral carbon atom?
What does it mean if a molecule is non-superimposable on its mirror image?
What does it mean if a molecule is non-superimposable on its mirror image?
Which terms describe the mirror image of a chiral molecule?
Which terms describe the mirror image of a chiral molecule?
According to Cahn-Ingold-Prelog (CIP) rules, what signifies an R configuration around a chiral carbon?
According to Cahn-Ingold-Prelog (CIP) rules, what signifies an R configuration around a chiral carbon?
Which of the following statements is true about the nature of chirality?
Which of the following statements is true about the nature of chirality?
In distinguishing chirality, which is not a method?
In distinguishing chirality, which is not a method?
Which of the following groups is critical in determining the priority in CIP rules?
Which of the following groups is critical in determining the priority in CIP rules?
Which statement correctly describes the relationship between chiral and achiral molecules?
Which statement correctly describes the relationship between chiral and achiral molecules?
What are enantiomers commonly associated with in chemical behavior?
What are enantiomers commonly associated with in chemical behavior?
Which of the following compounds is known to be an antibiotic?
Which of the following compounds is known to be an antibiotic?
What does the presence of a single chiral carbon imply about a molecule?
What does the presence of a single chiral carbon imply about a molecule?
Which of these compounds is classified as an ACE inhibitor?
Which of these compounds is classified as an ACE inhibitor?
What type of test can be used to identify if a carbon is chiral?
What type of test can be used to identify if a carbon is chiral?
What is true about diastereomers?
What is true about diastereomers?
Flashcards
Alkene Geometry
Alkene Geometry
The specific arrangement of atoms in a molecule, particularly around a double bond, which determines its shape and properties.
Stereoisomerism
Stereoisomerism
A type of isomerism where molecules have the same atoms and bonds but differ in their spatial arrangement, making them non-superimposable.
Chiral Carbon
Chiral Carbon
A carbon atom that is bonded to four different groups, resulting in the molecule having a non-superimposable mirror image.
Racemic Mixture
Racemic Mixture
Signup and view all the flashcards
Enantiomers
Enantiomers
Signup and view all the flashcards
Diastereoisomers
Diastereoisomers
Signup and view all the flashcards
Optical Activity
Optical Activity
Signup and view all the flashcards
Stereochemistry
Stereochemistry
Signup and view all the flashcards
What is a chiral carbon?
What is a chiral carbon?
Signup and view all the flashcards
How are atoms prioritized in chiral centers?
How are atoms prioritized in chiral centers?
Signup and view all the flashcards
What is stereochemistry?
What is stereochemistry?
Signup and view all the flashcards
What is an enantiomer?
What is an enantiomer?
Signup and view all the flashcards
What is a racemic mixture?
What is a racemic mixture?
Signup and view all the flashcards
What is optical activity?
What is optical activity?
Signup and view all the flashcards
What is the R/S system?
What is the R/S system?
Signup and view all the flashcards
What is an active enantiomer?
What is an active enantiomer?
Signup and view all the flashcards
Achiral
Achiral
Signup and view all the flashcards
Cahn-Ingold-Prelog (CIP) rules
Cahn-Ingold-Prelog (CIP) rules
Signup and view all the flashcards
R configuration
R configuration
Signup and view all the flashcards
S configuration
S configuration
Signup and view all the flashcards
Dextrorotatory
Dextrorotatory
Signup and view all the flashcards
Levorotatory
Levorotatory
Signup and view all the flashcards
Meso Compound
Meso Compound
Signup and view all the flashcards
Diastereomers
Diastereomers
Signup and view all the flashcards
Stereoisomer
Stereoisomer
Signup and view all the flashcards
Isomer
Isomer
Signup and view all the flashcards
Priority 4 in Plane
Priority 4 in Plane
Signup and view all the flashcards
Rotating for Chirality
Rotating for Chirality
Signup and view all the flashcards
Enantiomers and PPL Rotation
Enantiomers and PPL Rotation
Signup and view all the flashcards
Dextrorotatory (+) vs. Levorotatory (-)
Dextrorotatory (+) vs. Levorotatory (-)
Signup and view all the flashcards
Rotation and Configuration
Rotation and Configuration
Signup and view all the flashcards
Three-Point Model of Binding
Three-Point Model of Binding
Signup and view all the flashcards
Chiral Receptors and Enzymes
Chiral Receptors and Enzymes
Signup and view all the flashcards
Enantiomer Interactions
Enantiomer Interactions
Signup and view all the flashcards
Chiral Nitrogen Inversion
Chiral Nitrogen Inversion
Signup and view all the flashcards
Nitrogen Resonance and Chirality
Nitrogen Resonance and Chirality
Signup and view all the flashcards
What are enantiomers?
What are enantiomers?
Signup and view all the flashcards
Optical Activity of Enantiomers
Optical Activity of Enantiomers
Signup and view all the flashcards
Consequences of Inactive Enantiomers
Consequences of Inactive Enantiomers
Signup and view all the flashcards
Rotation of Plane-Polarized Light
Rotation of Plane-Polarized Light
Signup and view all the flashcards
Chirality in Pharmaceuticals
Chirality in Pharmaceuticals
Signup and view all the flashcards
What is the active enantiomer of Vigabatrin?
What is the active enantiomer of Vigabatrin?
Signup and view all the flashcards
Which enantiomer of Fluoxetine is considered the pharmacologically active form?
Which enantiomer of Fluoxetine is considered the pharmacologically active form?
Signup and view all the flashcards
Which enantiomer of Warfarin is more potent in inhibiting VKORC1?
Which enantiomer of Warfarin is more potent in inhibiting VKORC1?
Signup and view all the flashcards
Do all pairs of enantiomers display different activities?
Do all pairs of enantiomers display different activities?
Signup and view all the flashcards
Why is it critical to distinguish between undesirable activity and no adverse activity in enantiomers?
Why is it critical to distinguish between undesirable activity and no adverse activity in enantiomers?
Signup and view all the flashcards
How do active and inactive enantiomers differ in their interaction with receptors?
How do active and inactive enantiomers differ in their interaction with receptors?
Signup and view all the flashcards
Why are amines rarely chiral?
Why are amines rarely chiral?
Signup and view all the flashcards
Why does a racemic mixture exhibit no optical activity?
Why does a racemic mixture exhibit no optical activity?
Signup and view all the flashcards
What is meant by the 'pharmacologically active form' of a drug?
What is meant by the 'pharmacologically active form' of a drug?
Signup and view all the flashcards
What is chirality?
What is chirality?
Signup and view all the flashcards
Study Notes
Chirality (Abridged)
- This presentation covers chirality, a crucial concept in understanding pharmaceutical properties.
- Constitutional isomers, geometric isomers, and stereoisomerism are discussed.
- Constitutional isomers have the same molecular formula, but different connectivity of atoms.
- Different physical and chemical properties are observed.
- Constitutional isomers cannot interconvert.
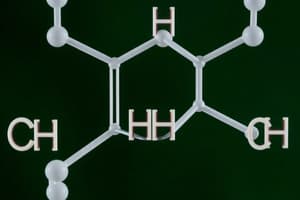
- Geometric isomers (cis-trans isomers) have the same molecular formula and connectivity, but different spatial arrangements of substituents around a double bond.
- Cis isomers have substituents on the same side of the double bond
- Trans isomers have substituents on opposite sides of the double bond
- No interconversion between cis and trans forms at ambient or body temperature.
- Examples include maleic acid (cis form) and fumaric acid (trans form).
- IUPAC nomenclature for alkenes uses Z (zusammen) and E (entgegen).
- Z = same side; E = opposite sides.
- Chiral centers are identified by considering the four different atoms or groups bonded to a carbon atom.
- If a molecule has a chiral center, its mirror image is not superimposable (non-superimposable mirror images).
- These are called enantiomers, and they have the same chemical properties, except for interaction with plane-polarized light (PPL).
- Plane-polarized light rotation is used to determine chiral molecules.
- Enantiomers rotate PPL in opposite directions by equal amounts.
- The presentation also highlights the importance of chirality in pharmaceuticals.
- The correct enantiomer is crucial for a drug's desired biological activity.
- Some examples of pharmaceuticals discussed are tamoxifen, aspirin, caffeic acid, 4-hydroxyphenylpyruvic acid.
- The different activities of enantiomers and their influence on drugs are covered.
- This is related to binding to receptors to trigger a biochemical response or not.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.