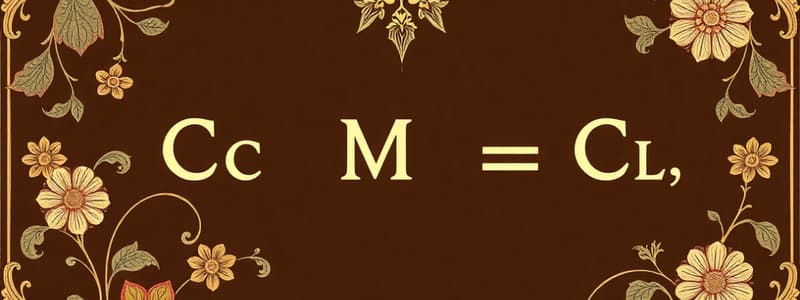Podcast
Questions and Answers
What is the correct name for CO₂?
What is the correct name for CO₂?
- Dicarbon oxide
- Carbon dioxide (correct)
- Carbon oxide
- Carbon monoxide
Charges must be balanced when writing formulas for covalent compounds.
Charges must be balanced when writing formulas for covalent compounds.
False (B)
What prefix is used for 'three' in naming compounds?
What prefix is used for 'three' in naming compounds?
tri
The formula for dinitrogen tetroxide is N₂O______.
The formula for dinitrogen tetroxide is N₂O______.
Match the following covalent compounds with their correct formulas:
Match the following covalent compounds with their correct formulas:
Which of the following describes an ionic compound?
Which of the following describes an ionic compound?
Covalent compounds require the use of Roman numerals to indicate the charges of the non-metals.
Covalent compounds require the use of Roman numerals to indicate the charges of the non-metals.
What is a cation?
What is a cation?
In naming ionic compounds, the anion's name is typically changed to end in _____ .
In naming ionic compounds, the anion's name is typically changed to end in _____ .
Match the following terms to their definitions:
Match the following terms to their definitions:
What is the correct chemical formula for Magnesium chloride?
What is the correct chemical formula for Magnesium chloride?
Ionic compounds usually consist of only non-metals.
Ionic compounds usually consist of only non-metals.
What is indicated by the Roman numeral in a compound like FeCl₃?
What is indicated by the Roman numeral in a compound like FeCl₃?
Match the following chemical formulas with their corresponding names:
Match the following chemical formulas with their corresponding names:
What is the chemical formula for potassium iodide?
What is the chemical formula for potassium iodide?
What is the chemical name for MgO?
What is the chemical name for MgO?
What is the chemical formula for aluminum chloride?
What is the chemical formula for aluminum chloride?
What is the chemical name for B₂H₄?
What is the chemical name for B₂H₄?
What is the chemical formula for sodium nitrate?
What is the chemical formula for sodium nitrate?
What is the chemical name for CaCO₃?
What is the chemical name for CaCO₃?
What is the chemical formula for nitrogen trichloride?
What is the chemical formula for nitrogen trichloride?
What is the chemical formula for phosphoric acid?
What is the chemical formula for phosphoric acid?
What is the chemical formula for sodium phosphate?
What is the chemical formula for sodium phosphate?
What is the chemical name for Cr(PO₄)₂?
What is the chemical name for Cr(PO₄)₂?
What is the chemical formula for aluminum carbonate?
What is the chemical formula for aluminum carbonate?
What is the chemical formula for phosphorus pentafluoride?
What is the chemical formula for phosphorus pentafluoride?
What is the chemical formula for magnesium acetate?
What is the chemical formula for magnesium acetate?
What is the chemical name for NH₄Cl?
What is the chemical name for NH₄Cl?
What is the chemical formula for magnesium hydroxide?
What is the chemical formula for magnesium hydroxide?
What is the chemical formula for iron(III) phosphate?
What is the chemical formula for iron(III) phosphate?
What is the chemical formula for lead(II) iodide?
What is the chemical formula for lead(II) iodide?
What is the chemical formula for nitrogen dioxide?
What is the chemical formula for nitrogen dioxide?
What is the chemical formula for sodium bromide?
What is the chemical formula for sodium bromide?
What is the chemical formula for sulfur trioxide?
What is the chemical formula for sulfur trioxide?
What is the chemical formula for copper(II) sulfate?
What is the chemical formula for copper(II) sulfate?
What is the chemical formula for beryllium hydroxide?
What is the chemical formula for beryllium hydroxide?
What is the chemical name for Ag₂(SO₃)?
What is the chemical name for Ag₂(SO₃)?
What is the chemical formula for carbon monoxide?
What is the chemical formula for carbon monoxide?
What is the chemical formula for nickel(III) hydroxide?
What is the chemical formula for nickel(III) hydroxide?
What is the chemical formula for sulfur hexafluoride?
What is the chemical formula for sulfur hexafluoride?
What is the chemical formula for phosphorus trihydride?
What is the chemical formula for phosphorus trihydride?
Flashcards
What is an Ionic Compound?
What is an Ionic Compound?
A compound formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions).
What are Ions?
What are Ions?
Atoms that have gained or lost electrons, resulting in a net positive or negative charge.
What is a Monatomic Ion?
What is a Monatomic Ion?
A single atom with a positive or negative charge. It's formed by the gain or loss of electrons.
What is a Polyatomic Ion?
What is a Polyatomic Ion?
Signup and view all the flashcards
What is a Covalent Compound?
What is a Covalent Compound?
Signup and view all the flashcards
What are the electrons doing in an ionic compound?
What are the electrons doing in an ionic compound?
Signup and view all the flashcards
What are the electrons doing in a covalent compound?
What are the electrons doing in a covalent compound?
Signup and view all the flashcards
How to determine the ratio between a cation and anion?
How to determine the ratio between a cation and anion?
Signup and view all the flashcards
Naming Order for Covalent Compounds
Naming Order for Covalent Compounds
Signup and view all the flashcards
Prefixes in Covalent Compound Names
Prefixes in Covalent Compound Names
Signup and view all the flashcards
Writing Formulas for Covalent Compounds
Writing Formulas for Covalent Compounds
Signup and view all the flashcards
Charges in Covalent Compounds
Charges in Covalent Compounds
Signup and view all the flashcards
Memorizing Polyatomic Ions
Memorizing Polyatomic Ions
Signup and view all the flashcards
Quale es le formula pro ioduro de potassio?
Quale es le formula pro ioduro de potassio?
Signup and view all the flashcards
Quale es le formula pro oxido de magnesio?
Quale es le formula pro oxido de magnesio?
Signup and view all the flashcards
Quale es le formula pro sulfide de stannio(II)?
Quale es le formula pro sulfide de stannio(II)?
Signup and view all the flashcards
Quale es le formula pro chloruro de aluminium?
Quale es le formula pro chloruro de aluminium?
Signup and view all the flashcards
Quale es le formula pro diboron tetrahydrido?
Quale es le formula pro diboron tetrahydrido?
Signup and view all the flashcards
Quale es le formula pro nitrato de natrium?
Quale es le formula pro nitrato de natrium?
Signup and view all the flashcards
Quale es le formula pro carbonato de calcio?
Quale es le formula pro carbonato de calcio?
Signup and view all the flashcards
Quale es le formula pro trichloruro de nitrogen?
Quale es le formula pro trichloruro de nitrogen?
Signup and view all the flashcards
Quale es le formula pro sulfato de lithium?
Quale es le formula pro sulfato de lithium?
Signup and view all the flashcards
Quale es le formula pro acido phosphoric?
Quale es le formula pro acido phosphoric?
Signup and view all the flashcards
Quale es le formula pro phosphide de beryllium?
Quale es le formula pro phosphide de beryllium?
Signup and view all the flashcards
Quale es le formula pro phosphate de natrium?
Quale es le formula pro phosphate de natrium?
Signup and view all the flashcards
Quale es le formula pro phosphate de chromium(VI)?
Quale es le formula pro phosphate de chromium(VI)?
Signup and view all the flashcards
Quale es le formula pro carbonato de aluminium?
Quale es le formula pro carbonato de aluminium?
Signup and view all the flashcards
Quale es le formula pro monoxide de nitrogen?
Quale es le formula pro monoxide de nitrogen?
Signup and view all the flashcards
Quale es le formula pro chloruro de calcio?
Quale es le formula pro chloruro de calcio?
Signup and view all the flashcards
Quale es le formula pro cyanide de natrium?
Quale es le formula pro cyanide de natrium?
Signup and view all the flashcards
Quale es le formula pro pentafluoruro de phosphoro?
Quale es le formula pro pentafluoruro de phosphoro?
Signup and view all the flashcards
Quale es le formula pro oxido de aluminium?
Quale es le formula pro oxido de aluminium?
Signup and view all the flashcards
Quale es le formula pro acetato de magnesio?
Quale es le formula pro acetato de magnesio?
Signup and view all the flashcards
Quale es le formula pro chloruro de ammonium?
Quale es le formula pro chloruro de ammonium?
Signup and view all the flashcards
Quale es le formula pro hydroxide de magnesio?
Quale es le formula pro hydroxide de magnesio?
Signup and view all the flashcards
Quale es le formula pro difluoruro de oxygen?
Quale es le formula pro difluoruro de oxygen?
Signup and view all the flashcards
Quale es le formula pro phosphate de ferro(III)?
Quale es le formula pro phosphate de ferro(III)?
Signup and view all the flashcards
Quale es le formula pro bromuro de magnesio?
Quale es le formula pro bromuro de magnesio?
Signup and view all the flashcards
Quale es le formula pro ioduro de plumb(II)?
Quale es le formula pro ioduro de plumb(II)?
Signup and view all the flashcards
Quale es le formula pro dinitrogen trisulfide?
Quale es le formula pro dinitrogen trisulfide?
Signup and view all the flashcards
Quale es le formula pro dioxido de nitrogen?
Quale es le formula pro dioxido de nitrogen?
Signup and view all the flashcards
Quale es le formula pro bromuro de natrium?
Quale es le formula pro bromuro de natrium?
Signup and view all the flashcards
Quale es le formula pro sulfide de lithium?
Quale es le formula pro sulfide de lithium?
Signup and view all the flashcards
Quale es le formula pro trioxido de sulfur?
Quale es le formula pro trioxido de sulfur?
Signup and view all the flashcards
Quale es le formula pro oxido de calcio?
Quale es le formula pro oxido de calcio?
Signup and view all the flashcards
Quale es le formula pro sulfato de cupro(II)?
Quale es le formula pro sulfato de cupro(II)?
Signup and view all the flashcards
Quale es le formula pro hydroxide de beryllium?
Quale es le formula pro hydroxide de beryllium?
Signup and view all the flashcards
Quale es le formula pro sulfite de argento?
Quale es le formula pro sulfite de argento?
Signup and view all the flashcards
Quale es le formula pro monoxide de carbon?
Quale es le formula pro monoxide de carbon?
Signup and view all the flashcards
Quale es le formula pro diphosphorus tetrabromide?
Quale es le formula pro diphosphorus tetrabromide?
Signup and view all the flashcards
Quale es le formula pro hydroxide de nickel(III)?
Quale es le formula pro hydroxide de nickel(III)?
Signup and view all the flashcards
Quale es le formula pro hexafluoruro de sulfur?
Quale es le formula pro hexafluoruro de sulfur?
Signup and view all the flashcards
Quale es le formula pro phosphate de ferro(III)?
Quale es le formula pro phosphate de ferro(III)?
Signup and view all the flashcards
Quale es le formula pro trifluoruro de boron?
Quale es le formula pro trifluoruro de boron?
Signup and view all the flashcards
Quale es le formula pro trihydrido de phosphoro?
Quale es le formula pro trihydrido de phosphoro?
Signup and view all the flashcards
Quale es le formula pro chlorato de stannio(II)?
Quale es le formula pro chlorato de stannio(II)?
Signup and view all the flashcards
Study Notes
Nomenclature Test - Study Guide
- Upcoming Test: Tuesday, November 17th.
- Study Guide Topics: Ions (cation/anion), monatomic ions, polyatomic ions, ionic compounds, covalent compounds (molecular compounds).
Ionic Compounds
- Formation: Formed between metals (cations) and nonmetals (anions).
- Electron Behavior: Electrons are transferred from the metal to the nonmetal, creating positively charged cations and negatively charged anions.
- Representative Elements: Metals from groups 1 and 2, and nonmetals from groups 16 and 17 (and others).
- Examples: Potassium iodide (KI), magnesium oxide (MgO), tin(II) sulfide (SnS), aluminum chloride (AlCl₃), sodium nitrate (NaNO₃), calcium carbonate (CaCO₃), lithium sulfate (Li₂SO₄), beryllium phosphide (Be₃P₂), sodium phosphate (Na₃PO₄), chromium (VI) phosphate (Cr(PO₄)₂), aluminum carbonate (Al₂(CO₃)₃), calcium chloride (CaCl₂), sodium cyanide (NaCN), magnesium bromide (MgBr₂), lead (II) iodide (PbI₂), magnesium acetate (Mg(CH₃CO₂)₂), ammonium chloride (NH₄Cl), magnesium hydroxide (Mg(OH)₂), copper(II) sulfate (CuSO₄), silver sulfite (Ag₂(SO₃)), iron (III) phosphate (FePO₄), aluminum oxide (Al₂O₃), calcium oxide (CaO)
Covalent Compounds (Molecular Compounds)
- Formation: Formed between nonmetals.
- Electron Behavior: Electrons are shared between atoms.
- Representative Elements: Nonmetals from various groups in the periodic table.
- Examples: Diboron tetrahydride (B₂H₄), nitrogen trichloride (NCl₃), nitrogen monoxide (NO), phosphorus pentafluoride (PF₅), oxygen difluoride (OF₂), dinitrogen trisulfide (N₂S₃), nitrogen dioxide (NO₂), sulfur trioxide (SO₃), carbon monoxide (CO), diphosphorus tetrabromide (P₂Br₄), sulfur hexafluoride (SF₆), boron trifluoride (BF₃), phosphorus trihydride (PH₃), tin (II) chlorate (Sn(ClO₃)₂)
Essential Skills for the Test
- Neutral Element Naming: Write the name of a neutral element.
- Neutral Element Formula: Determine the chemical formula of a neutral element.
- Compound Formula from Name (Ionic): Write the chemical formula of an ionic compound from its name. Examples: potassium iodide (KI), magnesium oxide (MgO), tin(II) sulfide (SnS), aluminum chloride (AlCl₃), sodium nitrate (NaNO₃).
- Compound Name from Formula (Ionic): Write the name of an ionic compound from its chemical formula. Examples: aluminum chloride (AlCl₃), sodium phosphate (Na₃PO₄), chromium (VI) phosphate (Cr(PO₄)₂)
- Monatomic/Polyatomic Ion Naming: Write the name for monatomic and polyatomic ions.
- Monatomic/Polyatomic Ion Formula from Name: Determine the chemical formula of monatomic or polyatomic ions from their names.
- Ion Charge Prediction: Predict the charge of an ion based on its position on the periodic table.
- Cation/Anion Ratio Prediction: Predict the ratio of cation to anion needed for a neutral compound.
Ionic Compound Naming (Binary)
- Method: Name the cation first, then the anion (ending in "-ide").
- Example: NaCl = Sodium chloride
Ionic Compound Naming (Transition Metals)
- Method: Use Roman numerals to indicate the metal's charge (variable charge).
- Example: FeCl₃ = Iron(III) chloride, tin(II) sulfide (SnS)
Ionic Compound Naming (Polyatomic Ions)
- Method: Name the cation first, then the polyatomic ion (without changing its name).
- Example: NaNO₃ = Sodium nitrate
Ionic Compound Formula Writing
- Step 1: Determine the charges of the ions using the periodic table or polyatomic ion chart.
- Step 2: Balance the charges to reach a neutral compound (0 net charge).
- Step 3: Write the formula with the cation first, then the anion.
- Example: Mg²⁺ + 2Cl⁻ = MgCl₂
Covalent Compound Naming
- Method: Use prefixes (mono-, di-, tri-, etc.) to indicate the number of atoms of each element.
- Less Electronegative First: The less electronegative element is named first.
- Ide Ending: The second element's name ends in "-ide".
- Examples: CO = Carbon monoxide, CO₂ = Carbon dioxide, N₂O₄ = Dinitrogen tetroxide, nitrogen trichloride (NCl₃), phosphorus pentafluoride (PF₅),
Covalent Compound Formula Writing
- Method: Convert prefixes to subscripts in the formula.
- Example: Diphosphorus pentoxide = P₂O₅
Key Tips & Important Info
- Memorize Polyatomic Ions: A list of common polyatomic ions will NOT be provided.
- Periodic Table: Use the periodic table to predict ion charges.
- Practice: Work through many examples!
Example Problems (Updated):
- Ionic: Name KBr, Ca(NO₃)₂, Fe₂O₃, write Aluminum sulfate, Copper(I) oxide, magnesium acetate, ammonium chloride, beryllium hydroxide.
- Covalent: Name PCl₃, SF₆, Write Carbon tetrachloride, Dinitrogen pentoxide, phosphorus trihydride (PH₃), sulfur hexafluoride(SF₆), nitrogen dioxide (NO₂).
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.