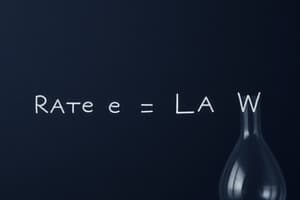Podcast
Questions and Answers
What does the order of reaction indicate?
What does the order of reaction indicate?
- The equilibrium state of a reaction
- The speed at which a reaction takes place
- The total amount of reactants and products in a reaction
- The relationship between the concentration of reactants and the rate of reaction (correct)
In a second-order reaction, if the concentration of reactant A is doubled, what happens to the reaction rate?
In a second-order reaction, if the concentration of reactant A is doubled, what happens to the reaction rate?
- It remains the same
- It is quadrupled
- It is halved
- It is doubled (correct)
What is the order of reaction with respect to a reactant?
What is the order of reaction with respect to a reactant?
- The temperature at which the reactant reacts
- The power to which the concentration of that reactant is raised in the rate equation (correct)
- The molar mass of the reactant
- The initial concentration of the reactant
What does molecularity refer to in a chemical reaction?
What does molecularity refer to in a chemical reaction?
In a unimolecular reaction, how many molecules are involved in the rate-determining step?
In a unimolecular reaction, how many molecules are involved in the rate-determining step?
What is the molecularity of a reaction if the rate equation is rate = k[A][B]?
What is the molecularity of a reaction if the rate equation is rate = k[A][B]?
Flashcards are hidden until you start studying