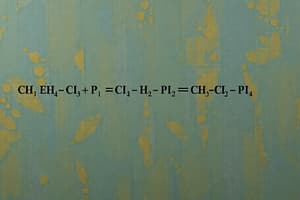Podcast
Questions and Answers
In which state are reactants and products present when they are dissolved in water?
In which state are reactants and products present when they are dissolved in water?
- Liquid state
- Solid state
- Aqueous solution state (correct)
- Gaseous state
How is the state of aqueous solution indicated in a chemical equation?
How is the state of aqueous solution indicated in a chemical equation?
- (aq) (correct)
- (g)
- [s]
- (l)
Which sign is used to indicate the requirement of external heat for a chemical reaction?
Which sign is used to indicate the requirement of external heat for a chemical reaction?
- (r) (correct)
- (+)
- (~)
- (?)
What does the presence of 'Heat' in a chemical equation signify?
What does the presence of 'Heat' in a chemical equation signify?
Which condition is usually indicated above or below the arrow in a chemical equation?
Which condition is usually indicated above or below the arrow in a chemical equation?
What does the symbol 'Ni Catalyst' represent in a chemical equation?
What does the symbol 'Ni Catalyst' represent in a chemical equation?
In a word equation, what is typically written below the formulae of reactants or products?
In a word equation, what is typically written below the formulae of reactants or products?
What does 'ZnSO4 (aq) + Cu (s) ZnSO4 (aq) + Cu (s) + Heat' represent?
What does 'ZnSO4 (aq) + Cu (s) ZnSO4 (aq) + Cu (s) + Heat' represent?
'CaCO3 (s) CaO (s) + CO2': Which type of reaction does this represent?
'CaCO3 (s) CaO (s) + CO2': Which type of reaction does this represent?
Flashcards are hidden until you start studying
Study Notes
Chemical Reaction and Equation
- A chemical reaction is a process where substances undergo bond breaking and are transformed into new substances by forming new bonds.
- Reactants are substances taking part in a chemical reaction, while products are substances formed as a result of a chemical reaction.
- A chemical reaction is represented by a chemical equation.
Conventions of Writing a Chemical Equation
- Reactants are written on the left-hand side, and products are written on the right-hand side.
- An arrow is drawn between the reactants and products, indicating the direction of the reaction.
- If there are multiple reactants or products, they are linked with a plus sign (+) in between them.
- The physical states of reactants are indicated in the equation using the letters (g), (l), and (s) for gaseous, liquid, and solid states, respectively.
Types of Chemical Equations
- A word equation is a simple way of representing a chemical reaction in words.
- A chemical equation is a representation of a chemical reaction in a condensed form using chemical formulae.
- Chemical equations can be further modified to indicate the physical states of reactants and products, heat, and conditions required for the reaction.
Examples of Chemical Equations
- CuSO4 + Zn → ZnSO4 + Cu
- CuSO4 (aq) + Zn (s) → ZnSO4 (aq) + Cu (s)
- CaCO3 (s) → CaO (s) + CO2 (heat is required)
- CuSO4 (aq) + Zn (s) → ZnSO4 (aq) + Cu (s) + Heat
- Vegetable oil (l) + H2 (g) → Vanaspathi ghee (s) (at 60°C, with Ni catalyst)
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.