Podcast
Questions and Answers
What is the primary role of mitochondria in the cell?
What is the primary role of mitochondria in the cell?
- DNA replication
- Protein synthesis
- Lipid metabolism
- Respiration and ATP synthesis (correct)
Which structure within the mitochondria is responsible for accumulating protons during the Electron Transport Chain?
Which structure within the mitochondria is responsible for accumulating protons during the Electron Transport Chain?
- Intermembrane space (correct)
- Outer membrane
- Matrix
- Inner membrane
What can be said about the mitochondria's genetic contributions to offspring?
What can be said about the mitochondria's genetic contributions to offspring?
- Only the mother provides mitochondria to the next generation (correct)
- Both parents contribute equally to mitochondrial DNA
- Mitochondria are inherited from grandparents
- Only the father contributes mitochondria
Which statement about the double-membrane structure of mitochondria is true?
Which statement about the double-membrane structure of mitochondria is true?
Which component of the mitochondria can be compared to the cytoplasm?
Which component of the mitochondria can be compared to the cytoplasm?
Which of the following best describes substrate-level phosphorylation?
Which of the following best describes substrate-level phosphorylation?
Oxidative phosphorylation occurs independent of the electron transport chain.
Oxidative phosphorylation occurs independent of the electron transport chain.
What are the two types of phosphorylation involved in cellular respiration?
What are the two types of phosphorylation involved in cellular respiration?
In cellular respiration, NADH and FADH₂ are involved in the _________ which helps in producing ATP.
In cellular respiration, NADH and FADH₂ are involved in the _________ which helps in producing ATP.
Match the following compounds with their roles in cellular respiration:
Match the following compounds with their roles in cellular respiration:
What are the two mobile electron carriers in the electron transport chain?
What are the two mobile electron carriers in the electron transport chain?
The majority of the DNA that encodes for the complexes in the electron transport chain is located in the mitochondrial genome.
The majority of the DNA that encodes for the complexes in the electron transport chain is located in the mitochondrial genome.
Name the process that involves the synthesis of ATP through the electron transport chain.
Name the process that involves the synthesis of ATP through the electron transport chain.
Match the following complexes with their characteristics in the electron transport chain:
Match the following complexes with their characteristics in the electron transport chain:
Which type of electron carrier is known to have a reactive site in the isoalloxazine ring?
Which type of electron carrier is known to have a reactive site in the isoalloxazine ring?
Cytochromes contain heme groups which are linked to magnesium ions.
Cytochromes contain heme groups which are linked to magnesium ions.
What is the reduced form of ubiquinone called?
What is the reduced form of ubiquinone called?
The final electron acceptor in the electron transport chain is _______.
The final electron acceptor in the electron transport chain is _______.
Match the following electron carriers with their characteristics:
Match the following electron carriers with their characteristics:
Match the following compounds with their definitions:
Match the following compounds with their definitions:
What is the main function of the Flavin Mononucleotide (FMN) in Complex I?
What is the main function of the Flavin Mononucleotide (FMN) in Complex I?
Complex II pumps protons into the intermembrane space.
Complex II pumps protons into the intermembrane space.
What is formed when Succinate donates an electron during the process in Complex II?
What is formed when Succinate donates an electron during the process in Complex II?
In Complex I, the electron from NADH is transferred to _________ before reaching Ubiquinone.
In Complex I, the electron from NADH is transferred to _________ before reaching Ubiquinone.
Match the following components of Complex I and Complex II with their respective roles:
Match the following components of Complex I and Complex II with their respective roles:
What is the final product formed when electrons are transferred to oxygen during the process in Complex IV?
What is the final product formed when electrons are transferred to oxygen during the process in Complex IV?
Name the molecule that acts as the final electron acceptor in Complex IV.
Name the molecule that acts as the final electron acceptor in Complex IV.
In the transfer of electrons from cytochrome C to the copper molecule, the pathway follows: ____ > ____ > ____ > ____.
In the transfer of electrons from cytochrome C to the copper molecule, the pathway follows: ____ > ____ > ____ > ____.
Match the following inhibitors with their types:
Match the following inhibitors with their types:
What is the role of ubiquinol (QH2) in Complex III?
What is the role of ubiquinol (QH2) in Complex III?
Complex III accepts electrons only from Complex I.
Complex III accepts electrons only from Complex I.
Match the components of Complex III with their functions:
Match the components of Complex III with their functions:
What is the primary function of the F1 and F0 structures in ATP synthase?
What is the primary function of the F1 and F0 structures in ATP synthase?
In the binding-change mechanism of ATP synthase, what happens during the Tight Conformation (T)?
In the binding-change mechanism of ATP synthase, what happens during the Tight Conformation (T)?
How do the F0 and F1 components of ATP synthase interact with each other?
How do the F0 and F1 components of ATP synthase interact with each other?
What conformational state occurs first in the sequence during ATP synthesis?
What conformational state occurs first in the sequence during ATP synthesis?
What initiates the conformational change in ATP synthase allowing ligands to bind?
What initiates the conformational change in ATP synthase allowing ligands to bind?
What drives ATP production through chemiosmotic coupling?
What drives ATP production through chemiosmotic coupling?
What is the role of the proton motive force in ATP synthesis?
What is the role of the proton motive force in ATP synthesis?
What is chemiosmotic coupling primarily related to in cellular respiration?
What is chemiosmotic coupling primarily related to in cellular respiration?
Which structure facilitates the movement of protons back into the mitochondrial matrix?
Which structure facilitates the movement of protons back into the mitochondrial matrix?
Which of the following compounds is an example of a protonophore that decreases ATP production?
Which of the following compounds is an example of a protonophore that decreases ATP production?
The entry of lipophilic weak acids into the mitochondrial matrix does not affect ATP production.
The entry of lipophilic weak acids into the mitochondrial matrix does not affect ATP production.
What effect does the dissipation of the electrochemical gradient have on ATP synthesis?
What effect does the dissipation of the electrochemical gradient have on ATP synthesis?
Match the following protonophores to their relevant actions:
Match the following protonophores to their relevant actions:
Match the following cell components with their roles in energy production:
Match the following cell components with their roles in energy production:
Match the following components with their functions in brown fat:
Match the following components with their functions in brown fat:
Match the following activators and inhibitors of brown fat with their effects:
Match the following activators and inhibitors of brown fat with their effects:
What is the primary function of brown fat in the body?
What is the primary function of brown fat in the body?
Brown fat contains a greater number of __________ than white fat.
Brown fat contains a greater number of __________ than white fat.
Match the following components with their roles in brown fat:
Match the following components with their roles in brown fat:
Which co-substrate is NOT produced as a byproduct during one round of the citric acid cycle?
Which co-substrate is NOT produced as a byproduct during one round of the citric acid cycle?
The citric acid cycle produces 3 NADH and 1 FADH2 per Acetyl CoA.
The citric acid cycle produces 3 NADH and 1 FADH2 per Acetyl CoA.
What is the irreversible enzyme that converts isocitrate to alpha-ketoglutarate in the citric acid cycle?
What is the irreversible enzyme that converts isocitrate to alpha-ketoglutarate in the citric acid cycle?
During the citric acid cycle, each molecule of NADH generates _____ ATP through oxidative phosphorylation.
During the citric acid cycle, each molecule of NADH generates _____ ATP through oxidative phosphorylation.
Match the following components of the citric acid cycle with their corresponding functions:
Match the following components of the citric acid cycle with their corresponding functions:
Which of the following statements correctly describes the respiratory control regarding NAD+ and FAD levels?
Which of the following statements correctly describes the respiratory control regarding NAD+ and FAD levels?
An increase in H+ concentration gradient promotes ATP synthesis during oxidative phosphorylation.
An increase in H+ concentration gradient promotes ATP synthesis during oxidative phosphorylation.
What happens to stored energy levels when exercise is not regular?
What happens to stored energy levels when exercise is not regular?
When catabolism is __________, NADH and FADH2 levels increase.
When catabolism is __________, NADH and FADH2 levels increase.
Match the process with its effects:
Match the process with its effects:
What is the main role of the electron transport chain?
What is the main role of the electron transport chain?
Uncouplers enhance the connection between the electron transport chain and ATP synthesis.
Uncouplers enhance the connection between the electron transport chain and ATP synthesis.
The chemiosmotic hypothesis relates to the coupling of _______ to oxidative phosphorylation.
The chemiosmotic hypothesis relates to the coupling of _______ to oxidative phosphorylation.
Match the following processes or components with their functions:
Match the following processes or components with their functions:
Study Notes
Mitochondria Overview
- Known as the "powerhouse" of the cell, responsible for respiration.
- Key organelle involved in converting molecules into ATP, the energy currency of the cell.
- Plays a crucial role in ATP synthesis.
Mitochondrial Compartments
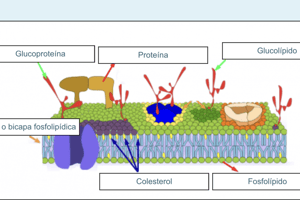
- Outer Membrane: Encloses the mitochondrion and acts as a barrier.
- Inner Membrane: Contains the majority of the enzymes of the Electron Transport Chain (ETC), vital for ATP production.
- Intermembrane Space: Area between the inner and outer membranes; protons accumulate here, creating a proton gradient necessary for ATP synthesis.
- Matrix: The inner liquid-filled portion, comparable to the cytoplasm of the cell, containing enzymes, DNA, and ribosomes.
- Cristae: Infoldings of the inner membrane, increasing surface area for chemical reactions.
Structural Characteristics
- Mitochondria have a double-membrane structure, composed of an outer and inner membrane.
- Similar to chloroplasts, which also feature a double-membrane system, supporting the endosymbiotic theory.
Endosymbiotic Theory
- Suggests that mitochondria originated from engulfed bacteria that predated eukaryotic cells.
- Supports the notion that both mitochondria and chloroplasts share a common ancestry via bacterial engulfment.
Mitochondrial Inheritance
- Mitochondria are maternally inherited; only the mother contributes mitochondrial DNA (mtDNA) to offspring.
- Mutations in the mitochondrial genome of the female can impact the health and function of the offspring.
- Nuclear genome mutations are less impactful due to the presence of DNA repair mechanisms.
Overview of Cellular Respiration
- Cellular respiration is the process by which sugar units (glucose) are converted into usable energy currency, ATP (adenosine triphosphate).
- This process occurs in the mitochondria of cells and involves various metabolic pathways.
Phosphorylation Types
-
Substrate-level phosphorylation
- Involves direct transfer of a phosphate group from one molecule to another.
- Example: GTP (guanosine triphosphate) is converted to ATP by transferring a phosphate to ADP (adenosine diphosphate).
-
Oxidative phosphorylation
- Occurs in the inner mitochondrial membrane, requiring the electron transport chain.
- Utilizes electrons from NADH and FADH₂ to facilitate ATP production.
Electron Transport Chain
- The electron transport chain comprises multiple complexes that facilitate electron transfer.
- NADH and FADH₂ donate electrons, which create a proton gradient across the mitochondrial membrane.
- The flow of protons back into the mitochondrial matrix drives ATP synthase, leading to ATP production.
Key Entities
- ATP: Main energy currency of the cell.
- NADH and FADH₂: Electron carriers produced during earlier stages of cellular respiration, such as glycolysis and the Krebs cycle.
- Complexes: Protein assemblies in the electron transport chain that facilitate the transfer of electrons and the subsequent generation of ATP.
Mitochondria and ATP Formation
- Mitochondria play a crucial role in ATP (adenosine triphosphate) production through the electron transport chain (ETC).
- The electron transport chain consists of four primary complexes: Complex I, Complex II, Complex III, and Complex IV, along with ATP Synthase.
- Four complexes are immobile integral membrane proteins, facilitating the transfer of electrons.
- Two mobile electron carriers, Ubiquinone and Cytochrome C, shuttle electrons between the complexes.
Genetic Encoding for ETC Complexes
- Specific DNA sequences encode the components of the electron transport chain.
- Most of this DNA is located in the nuclear genome, while some, such as Complex I, are found in the mitochondrial genome.
- Transcription of DNA serves as the initial step in creating the protein complexes essential for the ETC.
Protein Synthesis and Function
- Proteins required for the ETC process are primarily produced in the cytoplasm and ribosomes, often on the rough endoplasmic reticulum.
- The proteins synthesized outside the mitochondria are critical for the assembly and function of the electron transport chain.
Types of Electron Carriers
- Electron carriers play a crucial role in the electron transport chain (ETC) by accepting and donating electrons.
Flavoproteins
- Composed of polypeptides bound to either Flavin Adenine Dinucleotide (FAD) or Flavin Mononucleotide (FMN).
- FAD and FMN are key coenzymes in various biochemical reactions.
Structure of Flavoproteins
- Features a ribityl sugar backbone, which is a reduced form of ribose.
- Contains an isoalloxazine ring that serves as the reactive site.
Cytochromes
- Characterized by the presence of heme groups which contain iron (Fe) or copper (Cu) metal ions.
- The heme group consists of iron bound to a porphyrin ring, similar to hemoglobin.
- The reactive site in cytochromes is iron, existing in two states:
- Fe2+ (reduced form)
- Fe3+ (oxidized form)
Copper Atoms in Cytochromes
- Three copper atoms are integrated into a single protein complex, alternating between Cu2+ and Cu3+ oxidation states.
- Ceruloplasmin is an example of a copper-binding protein, playing a role in the oxidation of chiral-containing groups.
Ubiquinone (Coenzyme Q)
- A lipid-soluble molecule composed of five-carbon isoprenoid units.
- Exists in a reduced form known as ubiquinol.
- The conversion involves a transformation from ketone to alcohol.
- The reactive site is the carbonyl carbon, which has partial positive or negative charge properties.
Iron-Sulfur Proteins
- These proteins contain iron associated with inorganic sulfur, particularly from cysteine.
Arrangement in the Electron Transport Chain
- Electron carriers are organized by increasingly positive redox potential, facilitating efficient electron transfer.
- Key molecules in this arrangement include:
- Flavin mononucleotide (FMN)
- Coenzyme Q (ubiquinone)
- Cytochrome C
- Oxygen acts as the final electron acceptor in the chain.
End Process of Electron Transport
- The ultimate outcome of the ETC is the formation of water from the reduction of oxygen.
NAD+ and NADH
- NAD+ stands for nicotinamide adenine dinucleotide.
- It exists in an oxidized form, representing the molecule ready to accept electrons.
- NADH, or 1,4-dihydronicotinamide adenine dinucleotide, is the reduced form, containing additional electrons.
- The reactive site on NAD+ and NADH is indicated by a red circle, crucial for its role in biochemical reactions.
- Carbon 3 is important in the structure, playing a significant role in the functionality of these molecules.
FAD and FADH2
- FAD stands for flavin adenine dinucleotide.
- It exists in a reduced form known as FADH₂ after accepting electrons.
- The reactive site of FAD is the isoalloxazine ring, pivotal for its electron-carrying ability.
- Both NADH and FADH₂ are essential in metabolic processes, facilitating cellular respiration and energy production.
Complex I: NADH-CoQ Oxidoreductase
- Enzyme Classification: EC 1, an oxidoreductase enzyme.
- Structure: An integral membrane protein with both embedded and protruding parts in the membrane.
- Hydrophilic Domain: Faces aqueous matrix, contains the binding site for NADH.
- Electron Transfer: NADH donates an electron to Flavin Mononucleotide (FMN), producing NAD+ and FMNH.
- Iron-Sulfur Clusters: FMNH transfers electrons through multiple FeS clusters to ubiquinone, which is reduced to ubiquinol.
- Mitochondrial Orientation:
- Outer membrane
- Intermembrane space (proton-rich, positively charged)
- Inner membrane
- Matrix (negatively charged)
- Perspective Flip:
- Matrix ("paloob") faces inward
- Binding sites oriented outward
Complex II: Succinate-CoQ Oxidoreductase
- Alternate Name: Succinate Dehydrogenase.
- Electron Donor: Succinate transfers electrons to Flavin Adenine Dinucleotide (FAD), resulting in fumarate and FADH2 formation.
- Electron Transport: FADH2 donates electrons through a series of three iron-sulfur (FeS) clusters.
- Heme Function: Facilitates electron transport and anchorage within the complex.
- Endpoint of Transport: Coenzyme Q (CoQ) or Ubiquinone is reduced to Ubiquinol (QH2).
- Pumps and Electron Independence:
- Complex I electrons are distinct from Complex II electrons.
- Complex II does not pump protons; it is the only main complex that lacks this pumping mechanism.
- QH2 Transport: Both complexes (I and II) transfer QH2 through the mitochondrial membrane.
Complex III: CoQ-Cyt C Oxidoreductase
- Accepts electrons from both Complex 1 and Complex 2.
- Ubiquinol (QH2) delivers two electrons and protons to the complex; converts to Ubiquinone (CoQ).
- Ubiquinone cycles back to Complexes 1 and 2 for more electrons.
- Electrons are transferred via iron clusters to cytochrome-1.
- Cytochrome-1 transfers electrons to cytochrome c, reducing it for release into the intermembrane space.
- Structural changes in the complex occur with electron transfer, needing restoration to its initial state for repeated cycling.
- The lower part of Complex III is dedicated to maintenance and proton pumping.
- Essential to retain electrons through the electron transport pathway (ETP) from Complex 1 to Complex 4.
Complex IV: Cyt C Oxidase
- Serves as the final electron acceptor, essential for producing the final electron product.
- Reduced Cytochrome C donates electrons to Copper ions within the complex.
- Electrons cascade through Copper to Iron complexes (Cu > Fe > Fe > Cu).
- Final transfer of electrons from Copper to Oxygen occurs, resulting in the formation of water.
- Protons are simultaneously pumped during the electron transfer process.
- Oxygen is the oxidized form, while water represents the reduced form.
Inhibitors
- CO acts as a competitive inhibitor.
- CN− and H2S are noncompetitive inhibitors; caution against exposure to these substances.
- HCOOH (formic acid) and HN=N⁺=N⁻ are uncompetitive inhibitors.
Major Product
- The primary end product of this process is ATP synthesis.
Complex III Overview
- Complex III, also known as CoQ-Cyt C oxidoreductase, is a crucial component in the electron transport chain.
- It accepts electrons from both Complex I and Complex II.
Mechanism of Action
- Ubiquinol (QH2) delivers two electrons to Complex III, converting back to Ubiquinone (CoQ) in the process.
- The Ubiquinone then recycles back to Complexes I and II to acquire more electrons.
Electron Transfer Pathway
- The resident Ubiquinone in Complex III transfers electrons to iron clusters within the complex.
- These electrons ultimately reach cytochrome-1, which then transfers the electrons to cytochrome c.
- Cytochrome c gets reduced in the process and is released into the intermembrane space.
Structural Changes and Proton Pumping
- Electron transfer induces structural changes in the components of Complex III to ensure proper function.
- The lower part of the complex functions primarily for maintenance purposes and to facilitate proton pumping.
- The process of electron transfer is cyclic, crucially linking Complex I to Complex IV in the electron transport pathway.
ATP Synthase Structure
- Composed of five subunits: three alpha (ɑ), three beta (ꞵ), one delta (δ), one epsilon (ε), and one gamma (𝛾).
- Divided into two main parts:
- F1: Located outside the membrane, protruding into the matrix.
- F0: Embedded within the membrane, also referred to as the ATP turbine.
Functionality of ATP Synthase
- F0 rotates driven by protons, generating energy.
- F1 is rotated as a result of F0’s movement, with both segments rotating in opposite directions.
- The rotation mechanism enables ATP synthesis through a series of conformational changes.
Binding-Change Mechanism
- ATP synthase operates as a dimer in three distinct conformations:
- Open Conformation (O): No ligands (ADP and Pi) bound, preparing the active site for substrate entry.
- Loose Conformation (L): Ligands attach, inducing a structural change that loosely holds the substrates but does not join them.
- Tight Conformation (T): Allows ADP and Pi to combine, forming ATP.
- The sequence of conformations follows the cycle: O > L > T > T > O.
- The conformational changes are driven by the turbine-like rotation, allowing for substrate transition and ATP release.
Chemiosmotic Coupling
- ATP synthesis is linked to the movement of electrons and protons across the mitochondrial membrane.
- Protons accumulate in the intermembrane space, creating a proton motive force that drives ATP production.
Proton Gradient
- Cellular respiration generates a concentration gradient: high proton concentration in the intermembrane space and low concentration in the matrix.
- To restore equilibrium, protons move back into the matrix through the F0 component of ATP synthase.
ATP Production Mechanism
- The flow of protons back into the matrix powers ATP synthesis, illustrating the principle of chemiosmotic coupling.
- The process involves both F0 and F1 components of ATP synthase to enhance ATP production as protons flow from an area of high concentration to low concentration.
Mechanisms of Proton Transport
- Transport of H+ ions occurs across the inner mitochondrial membrane into the matrix, disrupting the electrochemical gradient.
- Discharging the electrochemical gradient halts ATP synthesis, as ATP production relies on H+ flow through ATP synthase.
- Lipophilic weak acids can enter the matrix and dissociate, leading to a decrease in free protons.
Key Compounds
- 2,4-Dinitrophenol (DNP) and carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) disrupt ATP synthesis by acting as protonophores.
- Protonophores facilitate proton transfer across membranes, allowing protons to bypass ATP synthase.
Effects on ATP Production
- The absence of protons in the matrix directly results in no ATP generation.
- The action of protonophores causes protons to return across the membrane without synthesizing ATP, resulting in energy loss as heat rather than being captured in ATP.
- The interplay between the use of protonophores and ATP synthesis illustrates the mechanisms of uncoupling in biological systems.
Mechanisms of Proton Transport
- Transport of H+ ions occurs across the inner mitochondrial membrane into the matrix, disrupting the electrochemical gradient.
- Discharging the electrochemical gradient halts ATP synthesis, as ATP production relies on H+ flow through ATP synthase.
- Lipophilic weak acids can enter the matrix and dissociate, leading to a decrease in free protons.
Key Compounds
- 2,4-Dinitrophenol (DNP) and carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) disrupt ATP synthesis by acting as protonophores.
- Protonophores facilitate proton transfer across membranes, allowing protons to bypass ATP synthase.
Effects on ATP Production
- The absence of protons in the matrix directly results in no ATP generation.
- The action of protonophores causes protons to return across the membrane without synthesizing ATP, resulting in energy loss as heat rather than being captured in ATP.
- The interplay between the use of protonophores and ATP synthesis illustrates the mechanisms of uncoupling in biological systems.
Mitochondria
- Organelles known as the powerhouses of the cell, pivotal for ATP production.
- Utilize a proton gradient generated during cellular respiration to synthesize ATP.
- The process occurs within the inner mitochondrial membrane via oxidative phosphorylation.
Chloroplasts
- Organelles found in plant cells responsible for photosynthesis.
- Create ATP using a mechanism similar to mitochondria, leveraging a proton gradient.
- The production of ATP is driven by sunlight through the process of photophosphorylation.
Bacteria (Respiration)
- Single-celled organisms also rely on proton gradients for energy production.
- Respiration in bacteria mirrors that of mitochondria, utilizing their cell membranes to create a proton motive force for ATP synthesis.
Photosynthetic Bacteria
- These bacteria perform photosynthesis similar to plants and chloroplasts.
- Generate a proton gradient during photosynthesis, crucial for ATP production.
- Utilize light energy to drive the production of ATP through processes involving their membrane structures.
Mitochondrial Uncoupling: Brown Fat vs. White Fat
- Brown fat is characterized by a higher number of mitochondria compared to white fat, contributing to its distinct brown color due to increased cytochrome content.
- Triacylglycerol (TAG) levels are elevated in brown fat, which aids in energy mobilization and heat production.
- Non-shivering thermogenesis occurs in brown fat, acting as a "biological heating pad" to generate heat in response to cold temperatures.
- Thermogenin, an uncoupling protein specific to brown fat, facilitates the regulated uncoupling of oxidative phosphorylation, thereby increasing heat production.
- Inhibition of thermogenesis can occur through ATP, ADP, GTP, and GDP, all of which can suppress the effectiveness of thermogenin.
- Activation of heat generation in brown fat is stimulated by free fatty acids, which are regulated by norepinephrine, a hormone that plays a key role in energy mobilization.
- Brown fat is crucial for thermoregulation, helping to maintain body temperature in cold environments by converting energy directly into heat.
- White fat primarily serves as energy storage and does not have the same thermogenic capabilities as brown fat, lacking the same density of mitochondria.
Mitochondrial Uncoupling: Brown Fat vs. White Fat
- Brown fat is characterized by a higher number of mitochondria compared to white fat, contributing to its distinct brown color due to increased cytochrome content.
- Triacylglycerol (TAG) levels are elevated in brown fat, which aids in energy mobilization and heat production.
- Non-shivering thermogenesis occurs in brown fat, acting as a "biological heating pad" to generate heat in response to cold temperatures.
- Thermogenin, an uncoupling protein specific to brown fat, facilitates the regulated uncoupling of oxidative phosphorylation, thereby increasing heat production.
- Inhibition of thermogenesis can occur through ATP, ADP, GTP, and GDP, all of which can suppress the effectiveness of thermogenin.
- Activation of heat generation in brown fat is stimulated by free fatty acids, which are regulated by norepinephrine, a hormone that plays a key role in energy mobilization.
- Brown fat is crucial for thermoregulation, helping to maintain body temperature in cold environments by converting energy directly into heat.
- White fat primarily serves as energy storage and does not have the same thermogenic capabilities as brown fat, lacking the same density of mitochondria.
Overview of the Citric Acid Cycle
- The cycle consists of key enzymes: citrate synthase, aconitase, isocitrate dehydrogenase (irreversible), α-ketoglutarate dehydrogenase (irreversible), succinate thiokinase, succinate dehydrogenase, fumarase, and malate dehydrogenase.
- Co-substrates involved in the cycle include 2 H2O, CoASH, and GDP + Pi.
- Byproducts generated include 2 CoASH, 2 CO2, and GTP.
- Each round of Acetyl CoA yields 3 NADH and 1 FADH2.
- The conversion factors for energy are: 1 NADH equals 2.5 ATP, while 1 FADH2 equals 1.5 ATP.
Connection to Oxidative Phosphorylation
- Initial state (catabolism off): Increased levels of NADH and FADH2, and decreased levels of NAD+ and FAD.
- Respiratory control phase involves elevated NAD+/FAD, signaling an activation of catabolism.
- Conversion processes lead to increased NADH and FADH2 which elevate the electron transport chain (ETC) activity.
- H+ concentration gradient increases as electrons are transported, promoting ATP synthesis via H+ influx into mitochondrial matrix.
- Resting state (catabolism off): Elevated NADH and FADH2 levels suggest high stored energy, leading to activation of anabolic processes if exercise is irregular.
Connection to Oxidative Phosphorylation
- Initial phase of catabolism results in increased levels of NADH and FADH2, while NAD+ and FAD concentrations decrease.
- Respiratory control involves the regulation of NAD+ and FAD levels, leading to an increase in NAD+ and FAD during active catabolism.
- In active catabolism, NADH and FADH2 are converted back to their oxidized forms, facilitating the electron transport chain (ETC).
- The ETC generates a hydrogen ion (H+) concentration gradient, which is essential for ATP synthesis.
- During rest periods, concentrations of NADH and FADH2 rise again, indicating a switch back to catabolism being inactive.
- If exercise is inconsistent or infrequent, energy reserves become high, prompting a shift towards anabolism (the building of complex molecules).
ATP Production in Cells
- ATP is generated through two main processes: substrate-level phosphorylation and oxidative phosphorylation.
- Oxidative phosphorylation involves the electron transport chain (ETC).
Electron Transport Chain (ETC)
- ETC consists of enzyme complexes that facilitate the transfer of electrons.
- Protons are transported across the mitochondrial membrane as electrons move through the chain.
Proton Gradient and ATP Synthesis
- The proton gradient created by the ETC is essential for the synthesis of ATP.
- This process of coupling the ETC to ATP production is explained by the chemiosmotic hypothesis.
Uncouplers and their Effects
- Uncouplers disrupt the link between the ETC and ATP synthesis.
- Disruption results in energy being released as heat instead of being stored as ATP.
Citric Acid Cycle and Reducing Equivalents
- Each molecule of acetyl CoA entering the Citric Acid Cycle yields 3 NADH and 1 FADH2.
- These reducing equivalents are crucial for energy production.
Regulation of Production During Exercise
- The production of reducing equivalents is regulated, particularly during physical activity.
- Insufficient regulation may lead to an accumulation of unutilized energy in the body.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.
Description
Explore the vital role of mitochondria, often referred to as the 'powerhouse' of the cell. This quiz covers its structure, compartments, and importance in ATP synthesis and respiration processes.