Podcast
Questions and Answers
Carbon dioxide in the atmosphere controls the fraction of outbound radiation.
Carbon dioxide in the atmosphere controls the fraction of outbound radiation.
False (B)
The concentration of total carbon dioxide in seawater is referred to as Total Alkalinity.
The concentration of total carbon dioxide in seawater is referred to as Total Alkalinity.
False (B)
The dissociation constant, $K_w$, for pure water decreases as the temperature increases from 0°C to 40°C.
The dissociation constant, $K_w$, for pure water decreases as the temperature increases from 0°C to 40°C.
True (A)
In low ionic solutions, pH is calculated as the positive log of the hydrogen ion concentration.
In low ionic solutions, pH is calculated as the positive log of the hydrogen ion concentration.
According to LeChatelier's Principle, changing the partial pressure affects the fractions of carbonic acid.
According to LeChatelier's Principle, changing the partial pressure affects the fractions of carbonic acid.
Carbonic anhydrase slows down hydration and dehydration reactions in respiratory organs.
Carbonic anhydrase slows down hydration and dehydration reactions in respiratory organs.
In the context of the carbonate system, $K_0$ represents the solubility constant.
In the context of the carbonate system, $K_0$ represents the solubility constant.
In the equation for $K_1$, increasing the concentration of $H_2CO_3$ will decrease the value of $K_1$.
In the equation for $K_1$, increasing the concentration of $H_2CO_3$ will decrease the value of $K_1$.
Total alkalinity in seawater is determined by measuring the concentration of hydroxide ions using a glass electrode.
Total alkalinity in seawater is determined by measuring the concentration of hydroxide ions using a glass electrode.
Borate alkalinity is a larger percentage of total alkalinity than carbonate alkalinity.
Borate alkalinity is a larger percentage of total alkalinity than carbonate alkalinity.
The concentrations of carbonate and phosphate ions are multiplied by a factor of 3 when calculating total alkalinity because they can accept three protons.
The concentrations of carbonate and phosphate ions are multiplied by a factor of 3 when calculating total alkalinity because they can accept three protons.
Carbonate Alkalinity is calculated by subtracting the contributions from borate and hydroxyl after Total Alkalinity measurements are completed.
Carbonate Alkalinity is calculated by subtracting the contributions from borate and hydroxyl after Total Alkalinity measurements are completed.
In surface seawater, the partial pressure can be calculated directly through alkalinity.
In surface seawater, the partial pressure can be calculated directly through alkalinity.
Adding a strong acid to seawater in a closed container will linearly decrease the pH.
Adding a strong acid to seawater in a closed container will linearly decrease the pH.
When acid is added to seawater, the system adjusts to reduce pH.
When acid is added to seawater, the system adjusts to reduce pH.
According to the solubility pump principle, CO2 exchange is independent of temperature.
According to the solubility pump principle, CO2 exchange is independent of temperature.
Calcium has a residence time that is replaced thousands of times.
Calcium has a residence time that is replaced thousands of times.
Aragonite is more stable than calcite, making it more abundant in marine sediments.
Aragonite is more stable than calcite, making it more abundant in marine sediments.
Forams contribute CaCO3 to deep oceans.
Forams contribute CaCO3 to deep oceans.
Coralline red algae is classified as aragonitic.
Coralline red algae is classified as aragonitic.
The thermodynamic constant, $K_{sp}$ is based on concentrations.
The thermodynamic constant, $K_{sp}$ is based on concentrations.
Aragonite has a higher the solubility and is therefore the more stable form.
Aragonite has a higher the solubility and is therefore the more stable form.
The degree of saturation is used to evaluate a solution that is over or saturated as compared to unsaturated.
The degree of saturation is used to evaluate a solution that is over or saturated as compared to unsaturated.
Activities are expressed in molarity.
Activities are expressed in molarity.
At high ion strength due to iron-ion interactions, ion concentration is greater than the ion activity.
At high ion strength due to iron-ion interactions, ion concentration is greater than the ion activity.
Increased salinity leads to higher consistency.
Increased salinity leads to higher consistency.
Solubility of calcium increases with increased salinity.
Solubility of calcium increases with increased salinity.
Calcium carbonate is commonly supersaturated at depths greater than 1000 meters.
Calcium carbonate is commonly supersaturated at depths greater than 1000 meters.
Lower Total CO2 in precipitation can cause a decrease in pCO2
Lower Total CO2 in precipitation can cause a decrease in pCO2
In an open system, the mitigation is increased when pH increases.
In an open system, the mitigation is increased when pH increases.
Atmospheric carbon dioxide concentrations show no seasonal variation.
Atmospheric carbon dioxide concentrations show no seasonal variation.
Based on ice-core data, CO₂ levels in the last 800,000 years never exceeded 200 ppm before recent times.
Based on ice-core data, CO₂ levels in the last 800,000 years never exceeded 200 ppm before recent times.
The rate of increase of CO₂ in the atmosphere over the last 60 years is about the same as previous natural increases on the geologic time scale.
The rate of increase of CO₂ in the atmosphere over the last 60 years is about the same as previous natural increases on the geologic time scale.
The largest reservoir of carbon on earth is organic carbon in sediments.
The largest reservoir of carbon on earth is organic carbon in sediments.
Rock weathering helps most of the Ca2+ get to the the sink.
Rock weathering helps most of the Ca2+ get to the the sink.
Surface pCO₂ tends to be higher during phytoplankton blooms due to respiration.
Surface pCO₂ tends to be higher during phytoplankton blooms due to respiration.
The biological pump transports organisms die and begin to sink to the ocean floor.
The biological pump transports organisms die and begin to sink to the ocean floor.
The biological pump moves fixed carbon to the sea floor, which may mineralized for primary reproduction.
The biological pump moves fixed carbon to the sea floor, which may mineralized for primary reproduction.
PCO₂ is typically lower in the North Pacific than in the North Atlantic at similar depths.
PCO₂ is typically lower in the North Pacific than in the North Atlantic at similar depths.
Alkalinity does not affect dissolution and formation of carbonate.
Alkalinity does not affect dissolution and formation of carbonate.
Flashcards
Why understand the CO₂ system?
Why understand the CO₂ system?
CO₂ controls the fraction of inbound radiation that remains trapped in the atmosphere, controlling planetary temperature.
CO₂ as an Anhydride
CO₂ as an Anhydride
CO₂ is an anhydride of carbonic acid, related by the loss/gain of water (H₂O).
What is Total Alkalinity (TA)?
What is Total Alkalinity (TA)?
Total Alkalinity is the sum of hydrogen ion equivalents of weak acid anions in solution and the total hydrogen ion neutralizing capacity of seawater.
Formula for Total Alkalinity
Formula for Total Alkalinity
Signup and view all the flashcards
Effect of Temperature on CO₂
Effect of Temperature on CO₂
Signup and view all the flashcards
Forams
Forams
Signup and view all the flashcards
Biological Precipitation
Biological Precipitation
Signup and view all the flashcards
Activity in Solubility
Activity in Solubility
Signup and view all the flashcards
Activity Coefficient
Activity Coefficient
Signup and view all the flashcards
Solubility vs Salinity
Solubility vs Salinity
Signup and view all the flashcards
Lysocline
Lysocline
Signup and view all the flashcards
Compensation Depth
Compensation Depth
Signup and view all the flashcards
CO₂ Increase
CO₂ Increase
Signup and view all the flashcards
Biological pump Steps
Biological pump Steps
Signup and view all the flashcards
What is ppm?
What is ppm?
Signup and view all the flashcards
Primary Production & pCO₂
Primary Production & pCO₂
Signup and view all the flashcards
Study Notes
- The topics include carbon dioxide, the carbonate system in seawater, calcium carbonate, solubility, precipitation and solution, anthropogenic carbon dioxide, and the distribution and behavior of carbonate species in the sea
Importance of Understanding the CO₂ System
- CO2 controls the fraction of inbound radiation trapped in the atmosphere, influencing planetary climate through the greenhouse effect
- CO2 serves as the raw material for building organic matter
- CO2 controls the pH of the oceans
- The distribution of CO2 species impacts the preservation of CaCO3 deposited on the sea floor
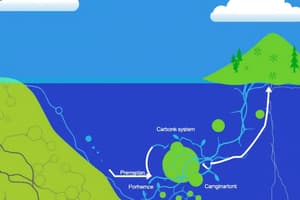
Carbon Dioxide and the Carbonate System
- CO2 is an anhydride of carbonic acid
- The chemical equation is CO2(f) + H2O ⇌ H2CO3 (carbonic acid)
- An anhydride is a chemical compound related to another through the loss/gain of water (H₂O)
Dissociations of Carbonic Acid
- The first dissociation is H2CO3 ⇌ H+ + HCO3- (bicarbonate)
- The second dissociation is HCO3- ⇌ H+ + CO3-2 (carbonate)
- The overall reaction illustrates the relationship between carbonic acid, bicarbonate, and carbonate ions in solution
- H2CO3 + CO3-2 ⇌ 2 HCO3- or CO2 + H2O + CO3-2 ⇌ 2 HCO3-
Parameters of the CO2 System in Seawater
- Understanding the CO2 system requires considering several key parameters:
- pH: A measure of the acidity or alkalinity of the seawater
- Alkalinity (TA): represents the buffering capacity of seawater against pH changes
- Total CO2 (TCO2 or DIC): the total concentration of all inorganic carbon species in the water
- Partial pressure of CO2 (pCO2): Measures the amount of CO2 dissolved in the water that is free to exchange with the atmosphere
About pH
- Water is a weak electrolyte and dissociates into a proton and a hydroxyl ion through the equation H₂O ⇌ H+ + OH-
- These ions combine with others to form hydronium ions (H3O+)
- The dissociation constant is Kw = {H+} {OH-} = 1.0 x 10-14 at 25°C in pure water
- pH uses a log scale, and glass electrodes respond linearly to the log of activity of the ion
- In low ionic solutions, pH = -log [H+], and in pure water at 25°C pH=7
- Kw changes with temperature and salinity, and at 0°C Kw = 7.47, at 40°C Kw = 6.76, at S=0‰ Kw = 7.0 and at S=40‰ Kw = 6.7
Fractions of Carbonic Acid as a function of pH
- LeChatelier's Principle can be applied when the pH is changed
- Seawater pH is ~7.9-8.4, with a surface water average of ~8.2
Hydration Rates
- CO₂ reacts slowly with water molecules
- The rate minimum occurs at intermediate pH values, relevant to seawater and biological fluids
- Carbonic anhydrase (enzyme) speeds these hydration and dehydration reactions in respiratory organs
Equilibria in Solution
- The constants K0, K1, and K2 describe equilibria involving CO2, carbonic acid, bicarbonate, and carbonate ions
- K0 relates CO2(f) + H2O to H2CO3
- K1 relates H2CO3 to HCO3 + H+
- K2 relates CO32- + H+
- Accessible parameters are temperature, salinity, pressure, {H+}, TCO2, [HCO3] + 2[CO32] (a) and ppCO2
Calculation of CO2 Species from pH, TCO2 & ppCO2
- The formula is expressed as TCO₂ = (ΣCO2) = [H2CO3] + [HCO3-] + [CO3-2]
- Concentrations of all CO2 species can be estimated from {H+} and ppCO2, {H+} and [TCO2] or from ppCO2 and [TCO2]
- Other terms include {H+} is activity of hydrogen ion (pH measurement), ppCO2 is partial pressure of CO2 (measure CO2 measure CO₂ in the gas phase) and [TCO2] is all forms of CO2 in solution (acidification and extraction measure extracted gas)
Alkalinity of Seawater
- Alkalinity refers to substances in seawater's ability to combine with hydrogen ions to protonate all carbonate species
- Total Alkalinity (TA) represents the sum of hydrogen ion equivalents of weak acid anions and equals the total hydrogen ion neutralizing capacity of seawater
- The major reactions when seawater is titrated with acid:
- CO32- + H+ → HCO3- HCO3- + H+ → H2CO3 = Carbonate alkalinity
- B(OH)4- + H+ → B(OH)3 + H20 Borate alkalinity
Boric Acid Equilibria
- Boric acid and borate ion form the second most important buffer system in seawater
- The equation is expressed as B(OH)3 + H2O ⇌ B(OH)4- + H+
- Boron is a conservative element in seawater, and its concentration is calculated based on the salinity ratio
- [TB] = [B(OH)4-] + [B(OH)3]
Back to Total Alkalinity
- TA can be calculated with the equation TA = CA + BA (Carbonate Alkalinity + Borate Alkalinity), and more extensively as TA = [HCO₃] + 2[CO₃-²] + [B(OH)₄⁻]
- The expanded formula that gives heightened accuracy is TA = [HCO₃] + 2[CO₃-²] + [B(OH)₄⁻] + [OH⁻] - [H⁺] + [SiO(OH)₃⁻] + [MgOH⁺] + [HPO₄-²] + 2[PO₄-³]
- Most of the terms is the longer equation are generally unimportant because of low concentration or low Ka
Why do [CO32-] And [PO43-] Have Coefficients of 2
- Coefficients relate to charge balance and contribution to neutralizing acid
- For carbonate: CO32- + 2H+ COOH (carbonic acid)
- Phosphates coefficient are ( tribasic phosphate to monobasic weak base
Alkalinity of Seawater
- Carbonate alkalinity (CA) is isolated from total by subtrating noncarbonates from:
- CA = [TA] - [B(OH)₄⁻] - [OH⁻] + [H⁺]
- Remainder after borate/hydroxal is:
- CA = [HCO₃⁻] + 2[CO₃-²]
Calculation of Carbonate Species from CA & PH
- TCO₂ formulas are: -(DIC, ΣCO2) = [H2CO3] + [HCO3] + [CO3-2] or - CA = 2 [CO3-2] + [HCO3-]
- [HCO₃] = [CA] x ({H+}/({H+}, -+2K2*)), [CO₃²] = [CA] ({K2*}/{H+}+2K2*) and [CO₂(aq)] = A({H2/Kc0 x K({}{H+}+2X2"))
Adding Acids In Equilibirum
- At equilibrium the system ajusts to (partically) counter act the effect of the appliekd change and establish a new equilibrium
- Carbonic acid dominates at pH less than 4.3
- Bicarbonate dominates at pH of 8.3
- Carbonate dominates at pH greater than 12.6
Effect of Temperature
- Temperature has a significant effect on the partial pressure of CO2, creating a major impact on CO2 exchange at the sea surface
- Temperature relationship plays a key role in oceanic CO2 transport as part of the solubility pump
Calcium Carbonate
- Calcium's residence time is approximately 675,000 years and is replaced 800-900x
- Entering the sea amounts to 21 x 1012 mol/y or 840 million tons
- Sedimentary records contain CaCO3 and CaMg(CO3)2, also known as dolomite
- CaCO3 precipitates form as Aragonite and Calcite
More on Calcium Carbonate
- Most precipitation is biological
- Forams, single-celled protozoa, form calcitic tests and contribute most significantly from plankton to the formation of sediments
- Coccoliths are single-celled planktonic algae that form calcitic plates and transport CaCO3 to deep oceans
- Molluscs form calcite and small planktonic preopods as aragonitic
- Coral reefs include coral animals and coralline red algae such as Lithothamnion & Porolithon
- Aragonitic may account for about 43# of Ca removal
- Algae - aragonitic Halimeda
- Segments can be a significant segment contributor around reef environments
- Inorganic precipatation - whitings formation in localized areas
- May precipate on organic nuclei
Calcium Carbonate and Solubility
- To assess the degree of saturation, compare the product of the product of concentrations of the various ions to the get a on product
- The equation is expressed as CaCO3 (s) ⇌ Ca2+ (aq) + CO32- (aq)
- Ksp (activities, not concentrations)
- In surface waters surface waters are ≈ 628 times supersaturated
Activity Coefficients
- Helps assess the degree of which a solution is over or under saturated
- For calcium carbonate the solubility product (Ksp) constants are approx 5.3x10-9 for Aragonite and 3.35x10-9 for Calcite
- Calcite has a lower solubility making it a more stable form
- Activities of the ratio of the ion produce and solubility product are YCa2+ = 0.256 and YCo32- = 0.205
- Surface waters are still greatly supersaturated in seawater
Ion Pairing
- Solubilty reduced by reduction in availability of free state
- Solubilty is (Ca2+) x YCa2+ free Ca )) (CO32-)* YC032"free CO3
- Organic molecules like Mg2+ may prevent CaCO2 nucleation
- Surfaces on waters can still be supersaturuated without correction
Calcium Carbonate Solubility
- Aragonite is more soluble the calcite being more stable
- Solubilty increases with salinity
- Salinity changes are sensistive to Calcium Carbonate
Solution of Calcium Carbonate
- Aragonite is more soluble than calcite
- The calcium carbonate compound is supersaturated at depths <500m
- Sediments of carbonite form at depths of >400m in Pacific
- Depth on the ocean at which the rates of dissolution greatly increace are called Lysocline
- Depth above carbonite can be preserved over rate of solvation is compensation depth
Effects of Solution and Precipitation
- Releases Co2 releases calcium carbonate
- There equation: Ca+2 +CaCO3 -> CaCO3
- No loss of Co2 replaced (to a point) HCo3- - CO32- + H+
- HCO3 + H+ → CO2 + H2O
- Formulas for TCO₂ and TA are -(DIC, ΣCO2) = [H2CO3] + [HCO3] + [CO3²] and TA =HCo₃ +2[CO3²]
- In total CaCO3 precipitation a CA reduces and pCO₂ increase
Effects of Solution And Precipitation
- PH decreases and PPco2 Increases in open sytem
- With the equation ATCo2 =0.760(A CA Co3)
Anathropogenic Carbon Dioxide
- Co2 in the atmospthere is a greenhouse gas; it absorbs infrared heat
- Concentrations vary both seasonally and geographically
Atmospheric Increase
- The increase over the last 60 years is 100 times faster than previous natural increases
- The atmospheric CO2 concentration in parts per million (ppm) dating back 800,00 years based on ice-core data (light purple line) compared to 2022
- Peaks and valleys in the line show ices ages, where CO2 was never hirer than 300ppm from 300,000 &400,000 years ago
Reservoirs of Carbon Dioxide
- Total Carbon in the Atmosphere and Crust of the Earth is in various forms that each form an important role
Climate Change And Sinks
- Sinks take up CO2 from what might otherwise be in the amtpsphere increasing the speed that atmoshpere levels are increasing
- Climlate Sinks :
- Rock weathing due to dissolusion
- Terrestrial uptake through Photosyntheiss rates still being uncertain
- Ocean Uptake depends on the factor which capcity decreases with more CO2 already abosribed
- These are the main types of feedback that help mitigate the greenhouse gas effects in long term
Distribution and Behavior of Carbonate Species in the Sea
- Carbonate concentration is controlled by the amount of increases and decreses
- This includes : --Photosynthesis decrease -Dissolusion -CO2 and Heat increase -Plat and material oxidation -Co3 Ca -And the amount that atmoshperes gas increases due to fossil fuels
Phytoplankton
- High primary production draws down surface pCO 2. As chlorophyll increases up, pCo2 decreases
The Biological carbon
- 1 Production: Production of fixed carbon by phytoplankton
- 2 Sinking - As Organism die and begin the ocean floor Sinking Rate
- 3 Remineralization fixed carbon is released
Ocean acidification
- PH profiles are oppisote to PCo2 because Co2 + H2O =H2Cro3
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.
Related Documents
Description
Explore the Carbon Dioxide and Carbonate System, its control over Earth's climate and the ocean's pH. Learn about the impact on calcium carbonate preservation and delve into the dissociations of carbonic acid in seawater. Understand the influence of anthropogenic carbon dioxide on the distribution of carbonate species.