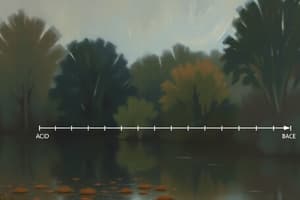Podcast
Questions and Answers
What happens to the pH of a buffer solution if the pH is greater than the pKa value?
What happens to the pH of a buffer solution if the pH is greater than the pKa value?
- pH becomes equal to pKa
- pH increases
- pH decreases (correct)
- pH remains constant
In a high (basic) environment, what is the state of ionization compared to unionization?
In a high (basic) environment, what is the state of ionization compared to unionization?
- IONIZED > UN-IONIZED (correct)
- IONIZED = UN-IONIZED
- Not enough information to determine
- IONIZED < UN-IONIZED
What percentage of molecules are ionized in a high (basic) environment?
What percentage of molecules are ionized in a high (basic) environment?
- 50%
- 100%
- 75% (correct)
- 25%
What is the property of a molecule that can cross a lipid membrane?
What is the property of a molecule that can cross a lipid membrane?
If the pH of a buffer solution is less than the pKa value, what happens to the pH?
If the pH of a buffer solution is less than the pKa value, what happens to the pH?
In a buffer system with a pH greater than the pKa value, what is the percentage of ionized molecules?
In a buffer system with a pH greater than the pKa value, what is the percentage of ionized molecules?
What happens to the pH of a buffer solution when it has a pH lower than the pKa value?
What happens to the pH of a buffer solution when it has a pH lower than the pKa value?
In which environment can molecules cross a lipid membrane?
In which environment can molecules cross a lipid membrane?
If a molecule is more than 50% ionized, what can be said about its state of unionization?
If a molecule is more than 50% ionized, what can be said about its state of unionization?
What is the relationship between the pH and pKa in a buffer system for the lipophilic property of a molecule?
What is the relationship between the pH and pKa in a buffer system for the lipophilic property of a molecule?
What is the relationship between ionization and unionization in a buffer system with a pH greater than the pKa value?
What is the relationship between ionization and unionization in a buffer system with a pH greater than the pKa value?
What is the likelihood of a molecule to cross a lipid membrane if it is more than 50% ionized?
What is the likelihood of a molecule to cross a lipid membrane if it is more than 50% ionized?
In a high (basic) environment, what happens to the percentage of ionized molecules?
In a high (basic) environment, what happens to the percentage of ionized molecules?
What occurs to a buffer solution's pH if it is less than the pKa value?
What occurs to a buffer solution's pH if it is less than the pKa value?
Which statement accurately describes the ability of molecules to cross a lipid membrane?
Which statement accurately describes the ability of molecules to cross a lipid membrane?
Flashcards are hidden until you start studying