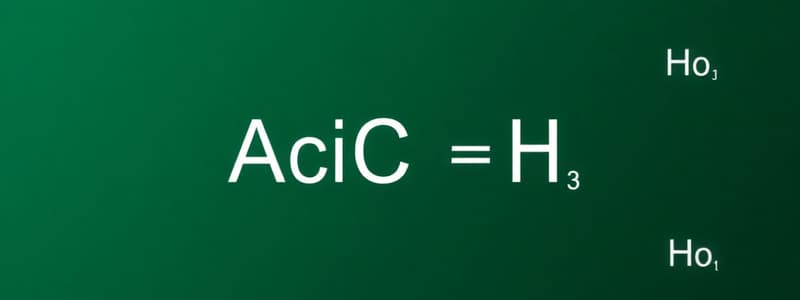Podcast
Questions and Answers
HCl represents Hydrochloric acid.
HCl represents Hydrochloric acid.
True (A)
HNO₃ is known as Sulfuric acid.
HNO₃ is known as Sulfuric acid.
False (B)
The formula for Hypobromic acid is HBrO₂.
The formula for Hypobromic acid is HBrO₂.
False (B)
Sulfurous acid is represented by the formula H₂SO₄.
Sulfurous acid is represented by the formula H₂SO₄.
Phosphorous acid is denoted by the formula H₃PO₃.
Phosphorous acid is denoted by the formula H₃PO₃.
Flashcards are hidden until you start studying
Study Notes
Acid Nomenclature - Part A:
- Hydrochloric acid is the name for the acid HCl(aq).
- Nitric acid is the name for the acid HNO₃(aq).
- Phosphoric acid is the name for the acid H₃PO₄(aq).
- Hydrosulfuric acid is the name for the acid H₂S(aq).
- Sulfuric acid is the name for the acid H₂SO₄(aq).
- Phosphorous acid is the name for the acid H₃PO₃(aq).
- Hydrofluoric acid is the name for the acid HF(aq).
- Acetic acid is the name for the acid CH₃COOH(aq).
- Perchloric acid is the name for the acid HClO₄(aq).
- Iodic acid is the name for the acid HIO₃(aq).
- Hydrobromic acid is the name for the acid HBr(aq).
- Hypobromous acid is the name for the acid HBrO₂(aq).
- Sulfurous acid is the name for the acid H₂SO₃(aq).
- Hypobromic acid is the name for the acid HBrO(aq).
Acid Nomenclature - Part B:
- Chromic acid is the formula H₂CrO₄.
- Sulfurous acid is the formula H₂SO₃.
- Phosphoric acid is the formula H₃PO₄.
- Hypochlorous acid is the formula HClO.
- Hydroiodic acid is the formula HI.
- Chlorous acid is the formula HClO₂.
- Nitrous acid is the formula HNO₂.
- Nitric acid is the formula HNO₃.
- Dichromic acid is the formula H₂Cr₂O₇.
- Bromic acid is the formula HBrO₃.
- Hydrosulfuric acid is the formula H₂S.
- Hydrobromic acid is the formula HBr.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.