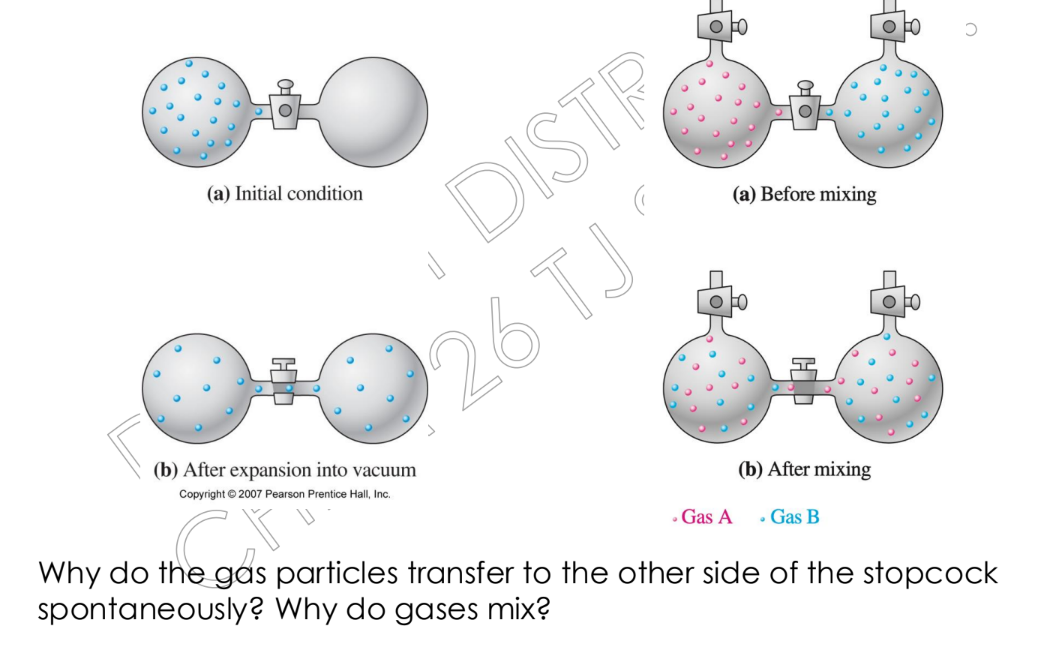
Why do gas particles transfer to the other side of the stopcock spontaneously? Why do gases mix?
Understand the Problem
The question is asking why gas particles transfer to the other side of a stopcock spontaneously and generally, why gases mix. This relates to concepts of entropy, diffusion, and the natural tendency of systems to increase disorder.
Answer
Gases mix because of diffusion, where molecules disperse due to concentration differences and thermal motion.
Gas particles move to the other side of the stopcock spontaneously and mix due to diffusion. Diffusion is the process by which molecules disperse in space because of differences in concentration and thermal motion of the gas particles.
Answer for screen readers
Gas particles move to the other side of the stopcock spontaneously and mix due to diffusion. Diffusion is the process by which molecules disperse in space because of differences in concentration and thermal motion of the gas particles.
More Information
Gases mix because gas molecules are in constant, random motion, and there is a natural tendency for them to spread out and occupy all available space. This is a spontaneous process driven by entropy (a measure of disorder) increasing as the gases mix.
Tips
A common mistake is thinking that gravity or other external forces significantly affect gas mixing in typical scenarios. While gravity does play a role in the atmosphere on a large scale, the dominant factor in small, closed systems is the constant random motion of the gas particles.
Sources
- 9.6 Effusion and Diffusion of Gases – Chemistry Fundamentals - pressbooks.online.ucf.edu
- 5.5: Effusion and Diffusion of Gases - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information