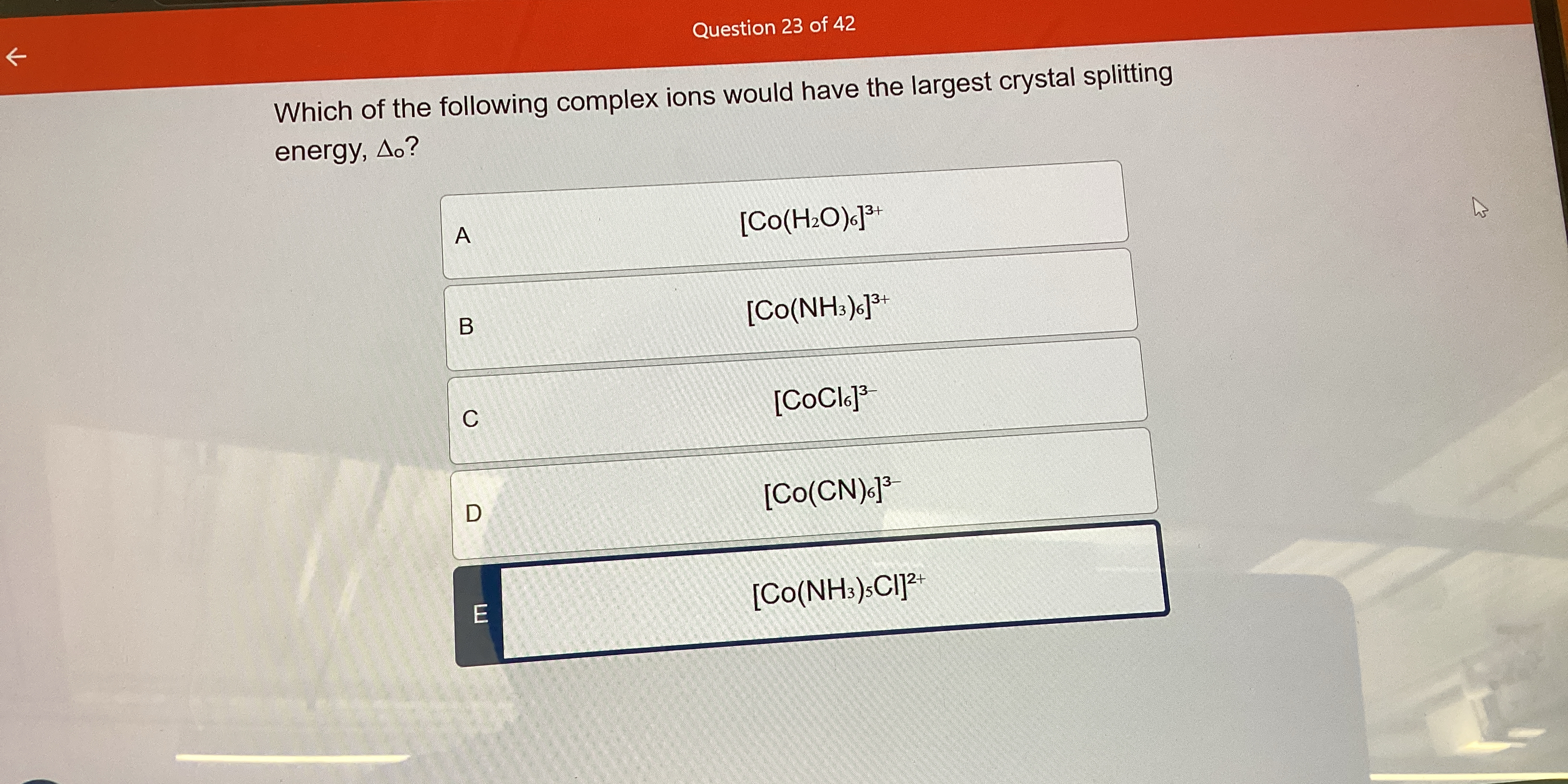
Which of the following complex ions would have the largest crystal splitting energy, Δo?
Understand the Problem
The question is asking us to identify which of the given complex ions would exhibit the largest crystal field splitting energy (Δo). This involves understanding the spectrochemical series and how different ligands affect the splitting of d-orbital energies in transition metal complexes.
Answer
The complex ion with the largest crystal field splitting energy is [Co(CN)6]3-.
The complex ion with the largest crystal field splitting energy, Δo, is D) [Co(CN)6]3-. This is because cyanide (CN-) is a strong field ligand.
Answer for screen readers
The complex ion with the largest crystal field splitting energy, Δo, is D) [Co(CN)6]3-. This is because cyanide (CN-) is a strong field ligand.
More Information
The spectrochemical series arranges ligands based on their ability to cause crystal field splitting. Strong field ligands like cyanide (CN-) cause a larger splitting than weak field ligands.
Tips
A common mistake is to not consider the spectrochemical series when determining the crystal field splitting energy. Remember to prioritize the ligand's position in the spectrochemical series.
Sources
- Solved Question 6 of 5 Which of the following complex ions - Chegg - chegg.com
- Expert-Verified Answer - brainly.com
- The complex that has the highest crystal splitting energy (Δ), is - toppr.com
AI-generated content may contain errors. Please verify critical information