Which compound would have the highest boiling point? a) CH3CH2CH2CH3 b) CH3CH2OCH3 c) CH3CH2CH2OH d) (CH3)2CHOH e) HOCH2CH2OH
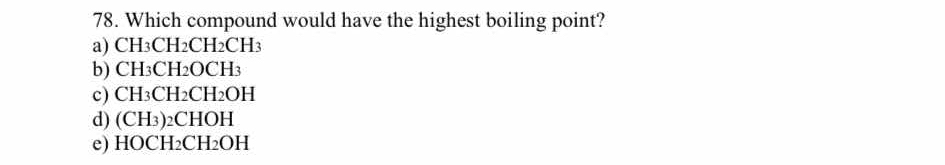
Understand the Problem
The question is asking which of the given compounds has the highest boiling point, based on their structure and functional groups.
Answer
HOCH2CH2OH
The compound with the highest boiling point is HOCH2CH2OH.
Answer for screen readers
The compound with the highest boiling point is HOCH2CH2OH.
More Information
Ethylene glycol (HOCH2CH2OH) has the highest boiling point due to strong hydrogen bonding provided by its two hydroxyl groups, which significantly increases its boiling point compared to other compounds.
Tips
A common mistake is underestimating the impact of hydrogen bonds. Always consider the number and strength of hydrogen bonds in a molecule.
Sources
- Which compound would have the highest boiling point ... - homework.study.com
- Which compound in each of the following pairs would have the ... - toppr.com
AI-generated content may contain errors. Please verify critical information