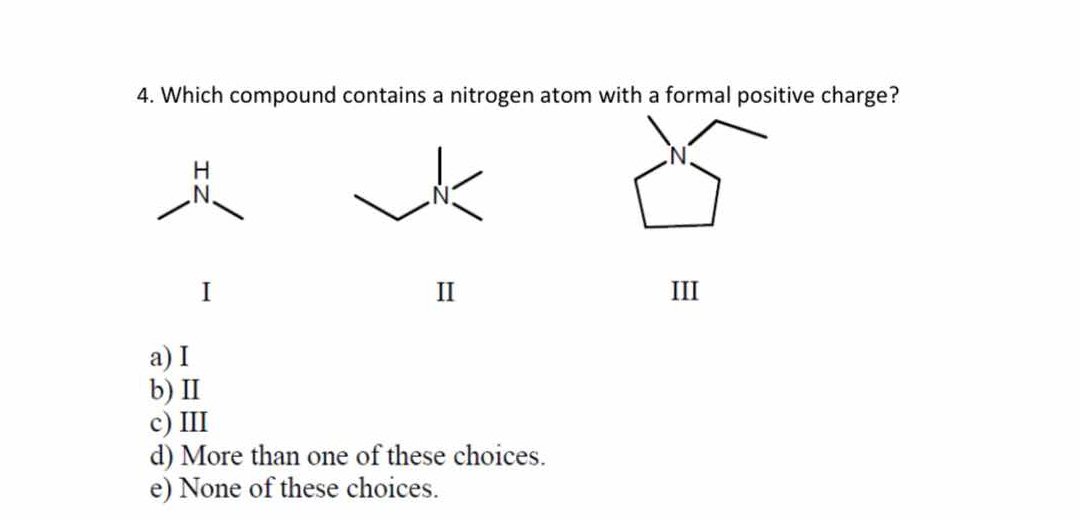
Which compound contains a nitrogen atom with a formal positive charge?
Understand the Problem
The question is asking which of the provided molecular structures contains a nitrogen atom that carries a formal positive charge. This involves analyzing the structures to determine the formal charge on the nitrogen atom in each compound.
Answer
Compound II
The compound II has a nitrogen atom with a formal positive charge.
Answer for screen readers
The compound II has a nitrogen atom with a formal positive charge.
More Information
Compound II contains a nitrogen atom bonded to three groups with no lone pairs, typically resulting in a formal positive charge.
Tips
A common mistake is not counting the electrons correctly. Ensure all bonding and non-bonding electrons are included.
Sources
- Formal Charges - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information