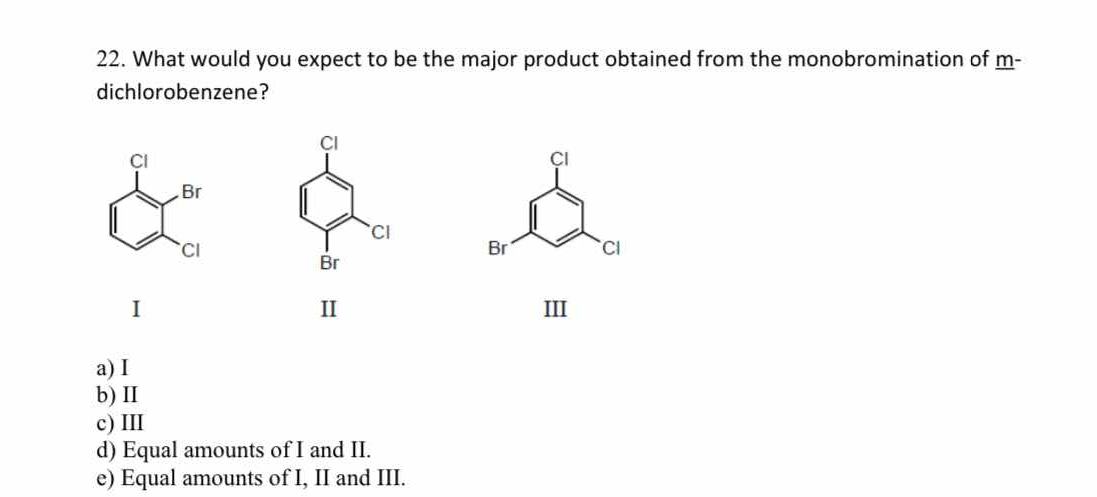
What would you expect to be the major product obtained from the monobromination of m-dichlorobenzene?
Understand the Problem
The question is asking about the expected major product from the monobromination of m-dichlorobenzene, which involves understanding the effects of existing substituents on the aromatic ring during electrophilic substitution reactions.
Answer
II
The final answer is II.
Answer for screen readers
The final answer is II.
More Information
In the case of m-dichlorobenzene, the chloro groups are electron-withdrawing and deactivate the positions ortho and para to them. However, the least hindered position para to neither of the chloro groups, which in a meta-substitution pattern would be the position ortho to one but para to none, is position II.
Tips
Confusing chloro as an activating group can lead to incorrect positioning of the electrophilic substitution.
Sources
AI-generated content may contain errors. Please verify critical information