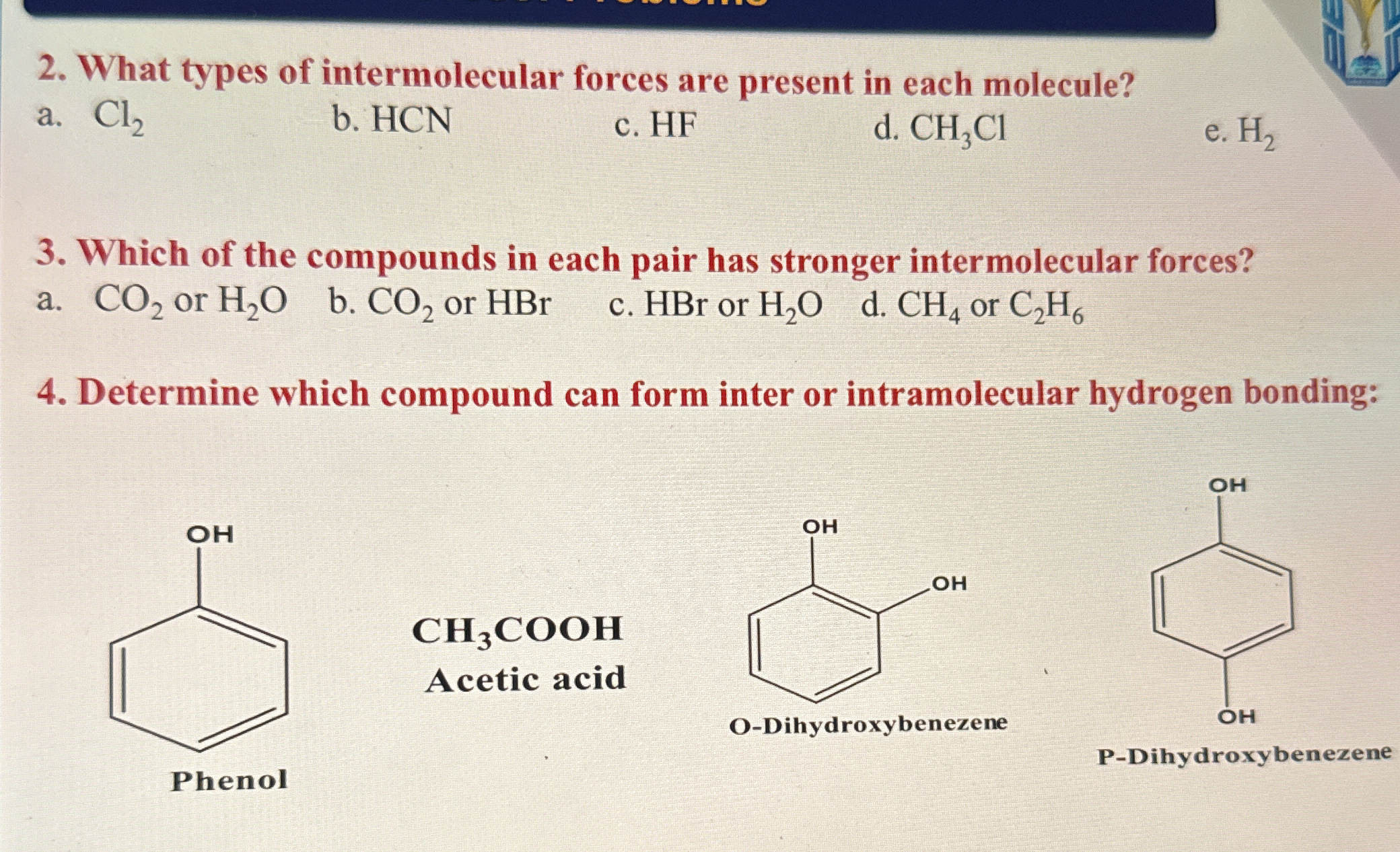
What types of intermolecular forces are present in each molecule? Which of the compounds in each pair has stronger intermolecular forces? Determine which compound can form inter or... What types of intermolecular forces are present in each molecule? Which of the compounds in each pair has stronger intermolecular forces? Determine which compound can form inter or intramolecular hydrogen bonding.
Understand the Problem
The question is asking about different types of intermolecular forces present in specified molecules, how to compare the strength of these forces in given pairs of compounds, and which compounds can engage in intra- or intermolecular hydrogen bonding. This involves concepts from intermolecular chemistry.
Answer
Cl₂, H₂: London dispersion; HCN: Dipole-dipole; HF: Hydrogen bonds; CH₃Cl: Dipole-dipole. Strongest: H₂O, HBr, H₂O, C₂H₆. Intramolecular: O-Dihydroxybenzene.
2a. Cl₂ - London dispersion forces. 2b. HCN - Dipole-dipole, hydrogen bonding. 2c. HF - Hydrogen bonding. 2d. CH₃Cl - Dipole-dipole. 2e. H₂ - London dispersion forces. 3a. H₂O has stronger forces (hydrogen bonding). 3b. HBr has stronger forces (dipole-dipole). 3c. H₂O has stronger forces (hydrogen bonding). 3d. C₂H₆ has stronger forces (slightly stronger London forces due to size). 4. O-Dihydroxybenzene can form intramolecular hydrogen bonding.
Answer for screen readers
2a. Cl₂ - London dispersion forces. 2b. HCN - Dipole-dipole, hydrogen bonding. 2c. HF - Hydrogen bonding. 2d. CH₃Cl - Dipole-dipole. 2e. H₂ - London dispersion forces. 3a. H₂O has stronger forces (hydrogen bonding). 3b. HBr has stronger forces (dipole-dipole). 3c. H₂O has stronger forces (hydrogen bonding). 3d. C₂H₆ has stronger forces (slightly stronger London forces due to size). 4. O-Dihydroxybenzene can form intramolecular hydrogen bonding.
More Information
Intermolecular forces significantly influence boiling and melting points. Stronger forces like hydrogen bonds result in higher boiling points compared to weak London dispersion forces.
Tips
Common mistake: Confusing dipole-dipole interactions with hydrogen bonding. Hydrogen bonding specifically requires hydrogen attached to nitrogen, oxygen, or fluorine.
Sources
- Intramolecular and intermolecular forces (article) - Khan Academy - khanacademy.org
- 11.2: Intermolecular Forces - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information