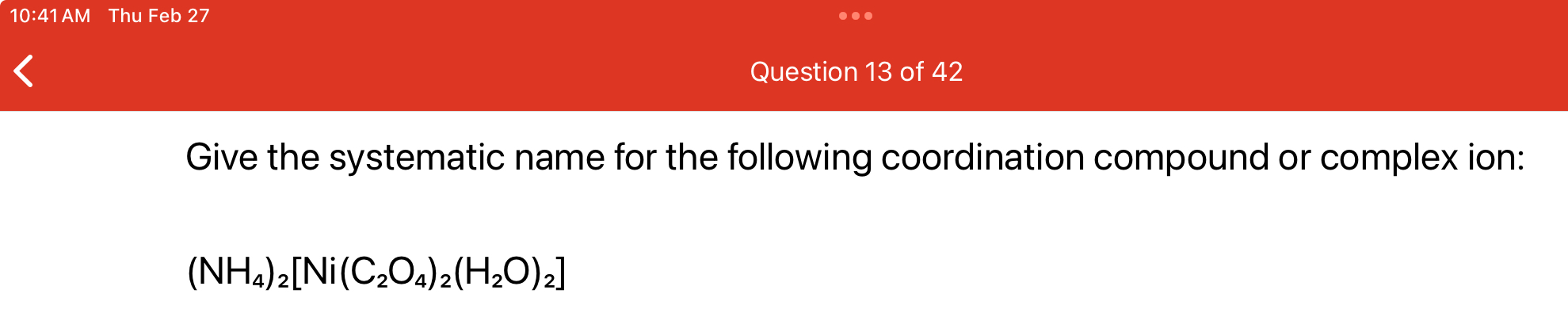
What is the systematic name for the coordination compound (NH4)2[Ni(C2O4)2(H2O)2]?
Understand the Problem
The question asks for the systematic name (IUPAC nomenclature) of the given coordination compound, (NH4)2[Ni(C2O4)2(H2O)2]. This involves identifying the ligands, their charges, and the oxidation state of the central metal ion (Nickel) to apply the correct naming conventions.
Answer
Ammonium diaquabis(oxalato)nickelate(II)
The systematic name for the coordination compound (NH4)2[Ni(C2O4)2(H2O)2] is ammonium diaquabis(oxalato)nickelate(II).
Answer for screen readers
The systematic name for the coordination compound (NH4)2[Ni(C2O4)2(H2O)2] is ammonium diaquabis(oxalato)nickelate(II).
More Information
The coordination compound consists of ammonium ions (NH4+) as counter ions and a complex ion containing a central nickel(II) ion coordinated by two oxalate (C2O4^2-) ligands and two water (H2O) ligands.
Sources
AI-generated content may contain errors. Please verify critical information