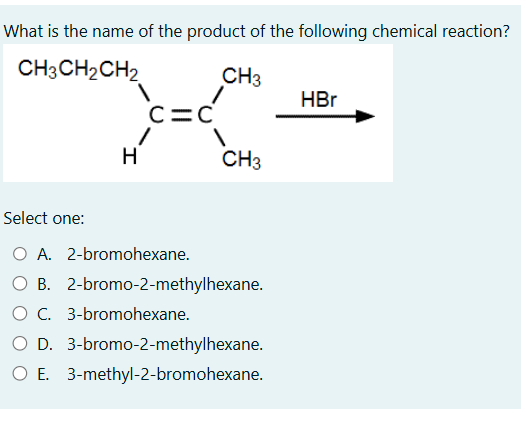
What is the name of the product of the following chemical reaction?
Understand the Problem
The question asks for the name of the product formed by the reaction of an alkene with HBr. This is an electrophilic addition reaction. According to Markovnikov's rule, the hydrogen atom will attach to the carbon with the higher number of hydrogen atoms, and the bromine atom will attach to the carbon with the fewer hydrogen atoms.
Answer
The final answer is D. 3-bromo-2-methylhexane.
The correct answer is D. 3-bromo-2-methylhexane.
Answer for screen readers
The correct answer is D. 3-bromo-2-methylhexane.
More Information
The reaction is an addition reaction of HBr to an alkene. The H atom adds to the carbon with more H atoms already (Markovnikov's rule), and the Br atom adds to the other carbon of the double bond.
Tips
Markovnikov's rule states that with the addition of a protic acid HX to an asymmetric alkene, the acid hydrogen (H) becomes attached to the carbon with more hydrogen substituents, while the halide (X) group becomes attached to the carbon with more alkyl substituents.
Sources
- Reactants & Products of a Chemical Reaction | Process & Examples - study.com
- 2.17: Reactants and Products - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information