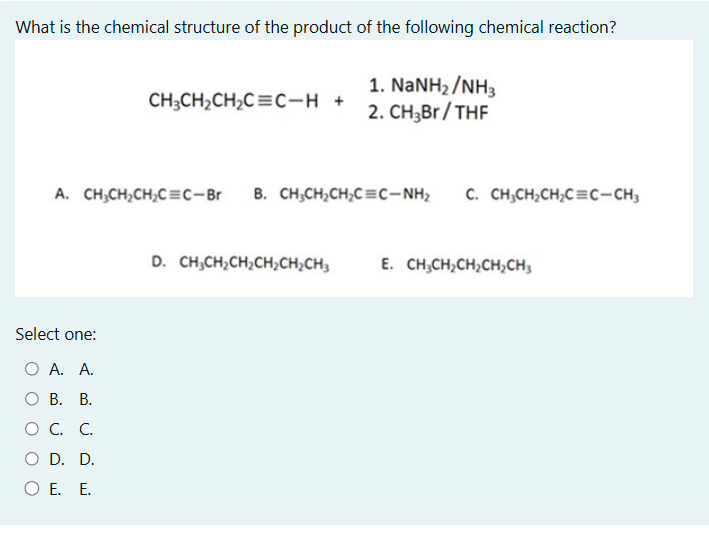
What is the chemical structure of the product of the following chemical reaction? CH3CH2CH2C≡C-H + (1) NaNH2/NH3 (2) CH3Br/THF
Understand the Problem
The question asks us to identify the final product of a two-step chemical reaction starting with an alkyne. The first step involves deprotonation using NaNH2 in NH3, followed by alkylation with CH3Br using THF as a solvent. We need to determine the structure of the resulting compound from the given options.
Answer
The answer is C. CH3CH2CH2C≡C-CH3 because the reaction sequence involves deprotonation of the terminal alkyne by NaNH2, followed by alkylation with CH3Br.
The final answer is C. CH3CH2CH2C≡C-CH3
Answer for screen readers
The final answer is C. CH3CH2CH2C≡C-CH3
More Information
The reaction is a sequence of deprotonation of a terminal alkyne followed by alkylation. NaNH2/NH3 is a strong base that removes the terminal proton, and CH3Br/THF adds a methyl group to the alkyne carbon.
Tips
A common mistake is to think that the Br adds directly to the alkyne. Remember that NaNH2 is a strong base and is used to deprotonate the terminal alkyne first.
Sources
- Solved Select the product formed from the following | Chegg.com - chegg.com
- CH3CH,CECH 1. NaNH2/NH3 2. CH3 CH2 CH2 CI/THF KMnO4 in ... - chegg.com
- [PDF] Fundamentals of Organic Chemistry - ChemistryDocs.Com - tech.chemistrydocs.com
AI-generated content may contain errors. Please verify critical information