What are the parts of an atom and their charges?
Understand the Problem
The question is asking for information on the composition and structure of atoms, detailing the roles of protons, neutrons, and electrons.
Answer
Atoms have protons (+), neutrons (no charge), and electrons (-).
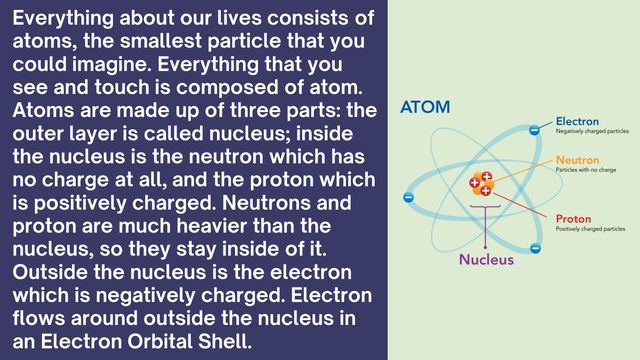
Atoms consist of protons, neutrons, and electrons. Protons have a positive charge and are located in the nucleus with neutrons, which have no charge. Electrons are negatively charged and orbit the nucleus.
Answer for screen readers
Atoms consist of protons, neutrons, and electrons. Protons have a positive charge and are located in the nucleus with neutrons, which have no charge. Electrons are negatively charged and orbit the nucleus.
More Information
Protons and neutrons form the nucleus and are roughly equal in mass. Electrons are much lighter and exist in orbitals around the nucleus.
Tips
A common mistake is confusing the charges of protons and electrons. Remember, protons are positive and electrons are negative.
Sources
- What is an Atom? - NRC - nrc.gov
- Atoms - ISBE PDF - isbe.net
- The Structure of the Atom - Introductory Chemistry - uen.pressbooks.pub
AI-generated content may contain errors. Please verify critical information