What are the key concepts related to gas mixtures, gas pressure, and ideal gas behavior based on the provided notes?
Understand the Problem
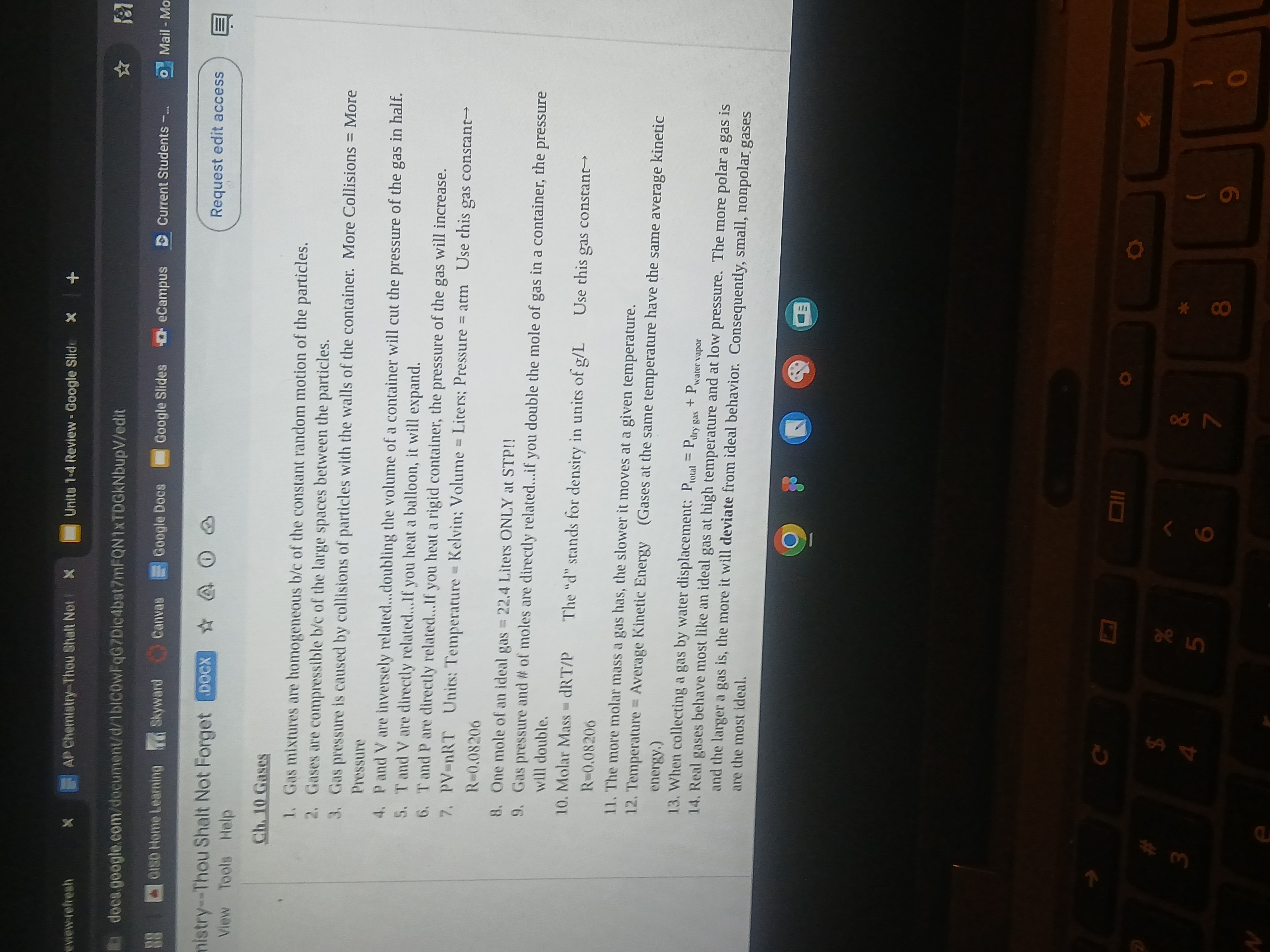
The image contains notes related to gas properties in chemistry, including definitions of gas mixtures, gas pressure, and the behavior of ideal gases. The question relates to understanding these concepts and potentially applying them to a chemistry problem.
Answer
Compressibility, ideal gas law, partial pressures, kinetic energy, deviation from ideal behavior.
Key concepts related to gas mixtures, gas pressure, and ideal gas behavior include: gases being compressible and homogeneous, gas pressure due to particle collisions, the ideal gas law (PV=nRT), Dalton’s Law of Partial Pressures, temperature's relationship with kinetic energy, and deviations from ideal behavior at low temperatures and high pressures.
Answer for screen readers
Key concepts related to gas mixtures, gas pressure, and ideal gas behavior include: gases being compressible and homogeneous, gas pressure due to particle collisions, the ideal gas law (PV=nRT), Dalton’s Law of Partial Pressures, temperature's relationship with kinetic energy, and deviations from ideal behavior at low temperatures and high pressures.
More Information
These concepts form the foundation of understanding gas behaviors in chemistry and physics, helping predict how gases will react under different conditions.
Tips
Not accounting for deviations from ideal gas behavior, especially at high pressures and low temperatures, can lead to incorrect predictions.
Sources
- 10.6: Gas Mixtures and Partial Pressures - Chemistry LibreTexts - chem.libretexts.org
- The Ideal Gas Law | Physics - Lumen Learning - courses.lumenlearning.com
AI-generated content may contain errors. Please verify critical information