What are the groups and classifications of elements in the periodic table?
Understand the Problem
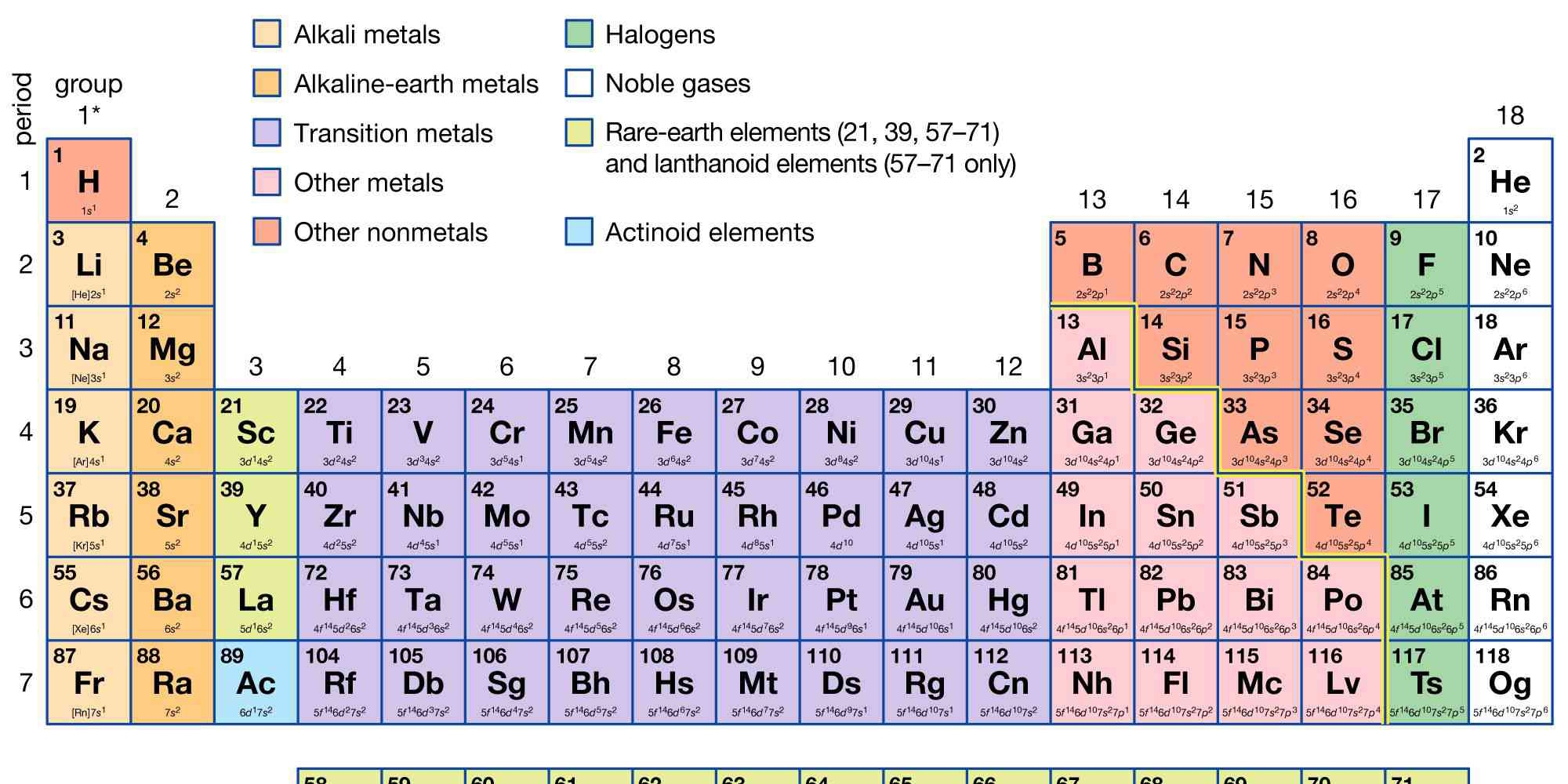
The question is asking for information or analysis related to the periodic table of elements, which includes the grouping, classification, and properties of various elements. This could involve identifying the categories or specific elements based on their location in the table.
Answer
Elements are grouped into 18 columns: alkali metals, alkaline-earth metals, transition metals, halogens, noble gases, and more.
Elements in the periodic table are grouped into 18 vertical columns known as groups. Key groups include alkali metals (group 1), alkaline-earth metals (group 2), transition metals (groups 3-12), and other main groups like halogens (group 17) and noble gases (group 18).
Answer for screen readers
Elements in the periodic table are grouped into 18 vertical columns known as groups. Key groups include alkali metals (group 1), alkaline-earth metals (group 2), transition metals (groups 3-12), and other main groups like halogens (group 17) and noble gases (group 18).
More Information
Groups in the periodic table categorize elements with similar chemical properties. For example, alkali metals are highly reactive, and noble gases are inert due to their complete electron shells.
Tips
A common mistake is confusing groups with periods. Groups are vertical columns, while periods are horizontal rows.
Sources
- Organization of the Periodic Table - Chemistry LibreTexts - chem.libretexts.org
- Group - Periodic Table of Elements: Los Alamos National Laboratory - periodic.lanl.gov
AI-generated content may contain errors. Please verify critical information