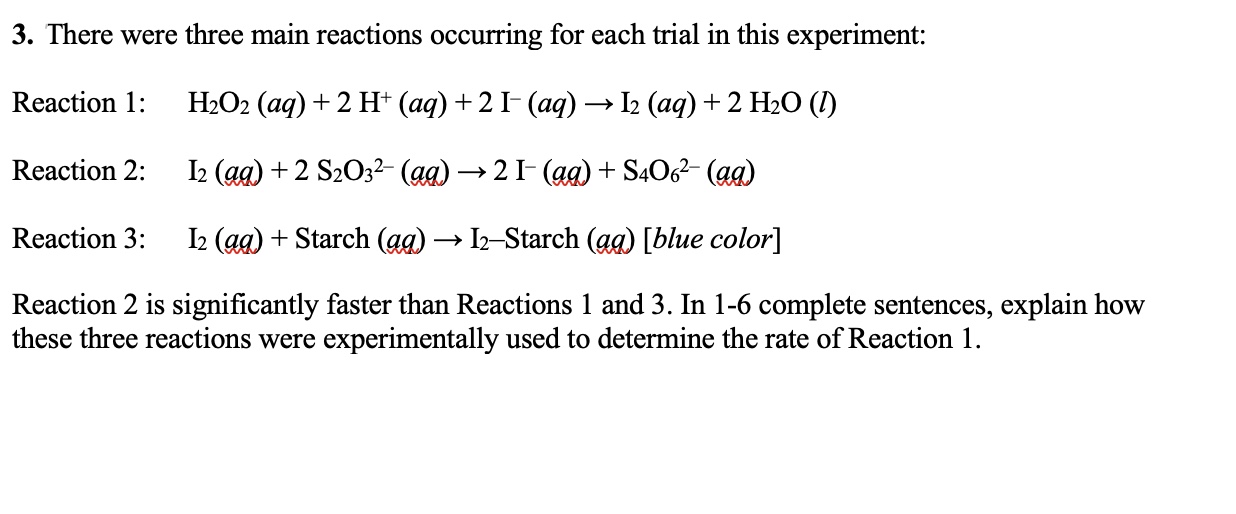
There were three main reactions occurring for each trial in this experiment: Reaction 1: H2O2 (aq) + 2 H+ (aq) + 2 I-(aq) → I2 (aq) + 2 H2O (l), Reaction 2: I2 (aq) + 2 S2O32- (aq)... There were three main reactions occurring for each trial in this experiment: Reaction 1: H2O2 (aq) + 2 H+ (aq) + 2 I-(aq) → I2 (aq) + 2 H2O (l), Reaction 2: I2 (aq) + 2 S2O32- (aq) → 2 I-(aq) + S4O62-(aq), Reaction 3: I2 (aq) + Starch (aq) → I2-Starch (aq) [blue color]. Reaction 2 is significantly faster than Reactions 1 and 3. In 1-6 complete sentences, explain how these three reactions were experimentally used to determine the rate of Reaction 1.
Understand the Problem
The question describes a series of three reactions used in an experiment. The goal is to explain how Reactions 2 and 3 are experimentally used in conjunction with Reaction 1 to determine the rate of Reaction 1. Understanding the rate-determining steps and the roles of the reactants in each reaction is essential to explain the experiment.
Answer
By measuring the time it takes for the solution to turn blue (indicating the consumption of a known amount of S2O32-), the rate of Reaction 1 can be determined.
Reaction 1 produces I2, which is immediately consumed by Reaction 2 (I2 + 2 S2O32- → 2 I- + S4O62-). A known amount of S2O32- is added, and once it is completely consumed by Reaction 2, any further I2 produced by Reaction 1 will react with starch (Reaction 3), creating a blue color. The time it takes for the blue color to appear is measured, which indicates the time it took for Reaction 1 to produce enough I2 to react with all the added S2O32-. Knowing the amount of S2O32- and the time elapsed, the rate of Reaction 1 can be determined.
Answer for screen readers
Reaction 1 produces I2, which is immediately consumed by Reaction 2 (I2 + 2 S2O32- → 2 I- + S4O62-). A known amount of S2O32- is added, and once it is completely consumed by Reaction 2, any further I2 produced by Reaction 1 will react with starch (Reaction 3), creating a blue color. The time it takes for the blue color to appear is measured, which indicates the time it took for Reaction 1 to produce enough I2 to react with all the added S2O32-. Knowing the amount of S2O32- and the time elapsed, the rate of Reaction 1 can be determined.
More Information
The experiment described is commonly known as an iodine clock reaction. It relies on the rapid consumption of iodine (I2) by thiosulfate ions (S2O32-) to delay the reaction of iodine with starch, which produces a visible blue color. The time it takes for the blue color to appear is an indirect measure of the rate of the initial reaction.
Tips
A common mistake is not understanding that the amount of thiosulfate (S2O32-) added is known and is completely consumed before the blue color appears. The rate of Reaction 1 is determined based on how long it takes to use up all the thiosulfate.
Sources
- 1 - The Iodine Clock Reaction - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information