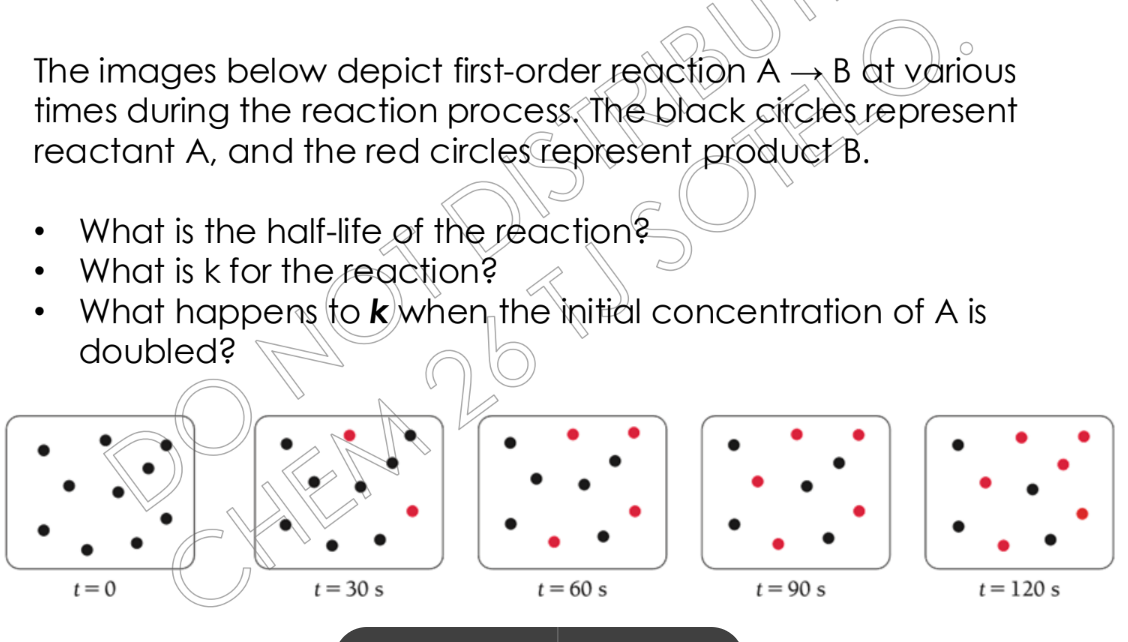
The images below depict first-order reaction A → B at various times during the reaction process. The black circles represent reactant A, and the red circles represent product B. W... The images below depict first-order reaction A → B at various times during the reaction process. The black circles represent reactant A, and the red circles represent product B. What is the half-life of the reaction? What is k for the reaction? What happens to k when the initial concentration of A is doubled?
Understand the Problem
The question presents a first-order reaction A -> B, showing the change in the number of reactant A and product B molecules at different time points. It asks to determine the half-life of the reaction, calculate the rate constant (k), and describe the effect of doubling the initial concentration of A on the rate constant. The black circles represent reactant A, and the red circles represent product B.
Answer
Half-life: 30 s, k: 0.0231 s⁻¹, k remains unchanged.
The half-life of the reaction is 30 seconds. k for the reaction is 0.0231 s⁻¹. k does not change when the initial concentration of A is doubled because it is a first-order reaction.
Answer for screen readers
The half-life of the reaction is 30 seconds. k for the reaction is 0.0231 s⁻¹. k does not change when the initial concentration of A is doubled because it is a first-order reaction.
More Information
For a first-order reaction, the rate constant (k) is independent of the initial concentration of the reactant. The formula k = ln(2) / t1/2 can be used to derive k.
Tips
A common mistake is thinking the rate constant changes with concentration for first-order reactions. Remember that k is constant for a given reaction at a specific temperature.
Sources
- 5.1: Determining Reaction Order - Chemistry LibreTexts - chem.libretexts.org
- 12.4: Rate Laws - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information