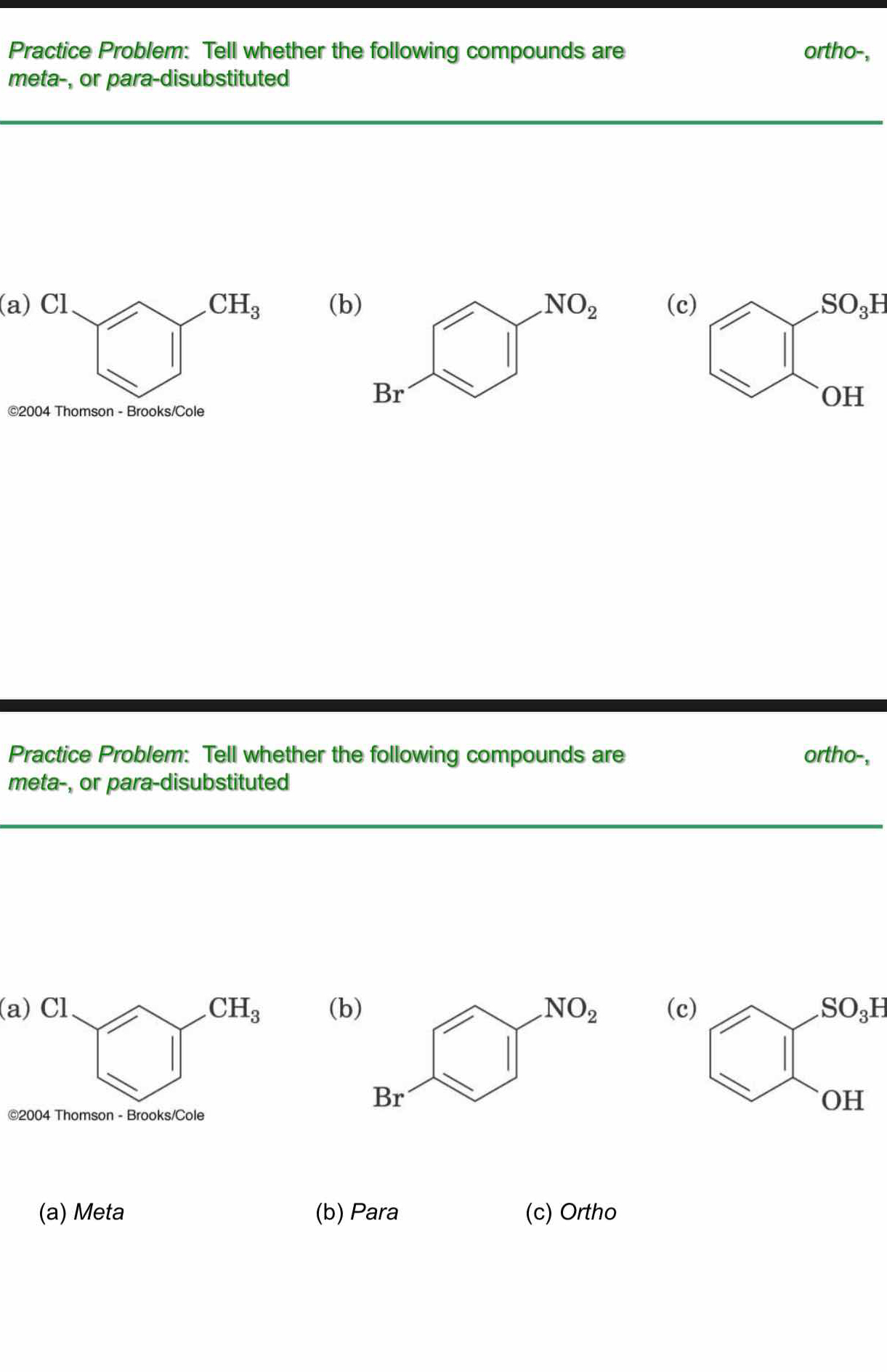
Tell whether the following compounds are ortho-, meta-, or para-disubstituted.
Understand the Problem
The question is asking to classify the given compounds based on their substitution pattern as either ortho-, meta-, or para-disubstituted. This requires an understanding of the positions of substituents on the benzene ring.
Answer
(a) Meta (b) Para (c) Ortho
(a) Meta (b) Para (c) Ortho
Answer for screen readers
(a) Meta (b) Para (c) Ortho
More Information
Ortho, meta, and para are terms used to describe the relative positions of substituents on a benzene ring. These terms help in identifying structural isomers.
Tips
A common mistake is confusing the positions of substituents. Remember the positions: 1,2 for ortho, 1,3 for meta, and 1,4 for para.
Sources
- 15.1: Naming Aromatic Compounds - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information