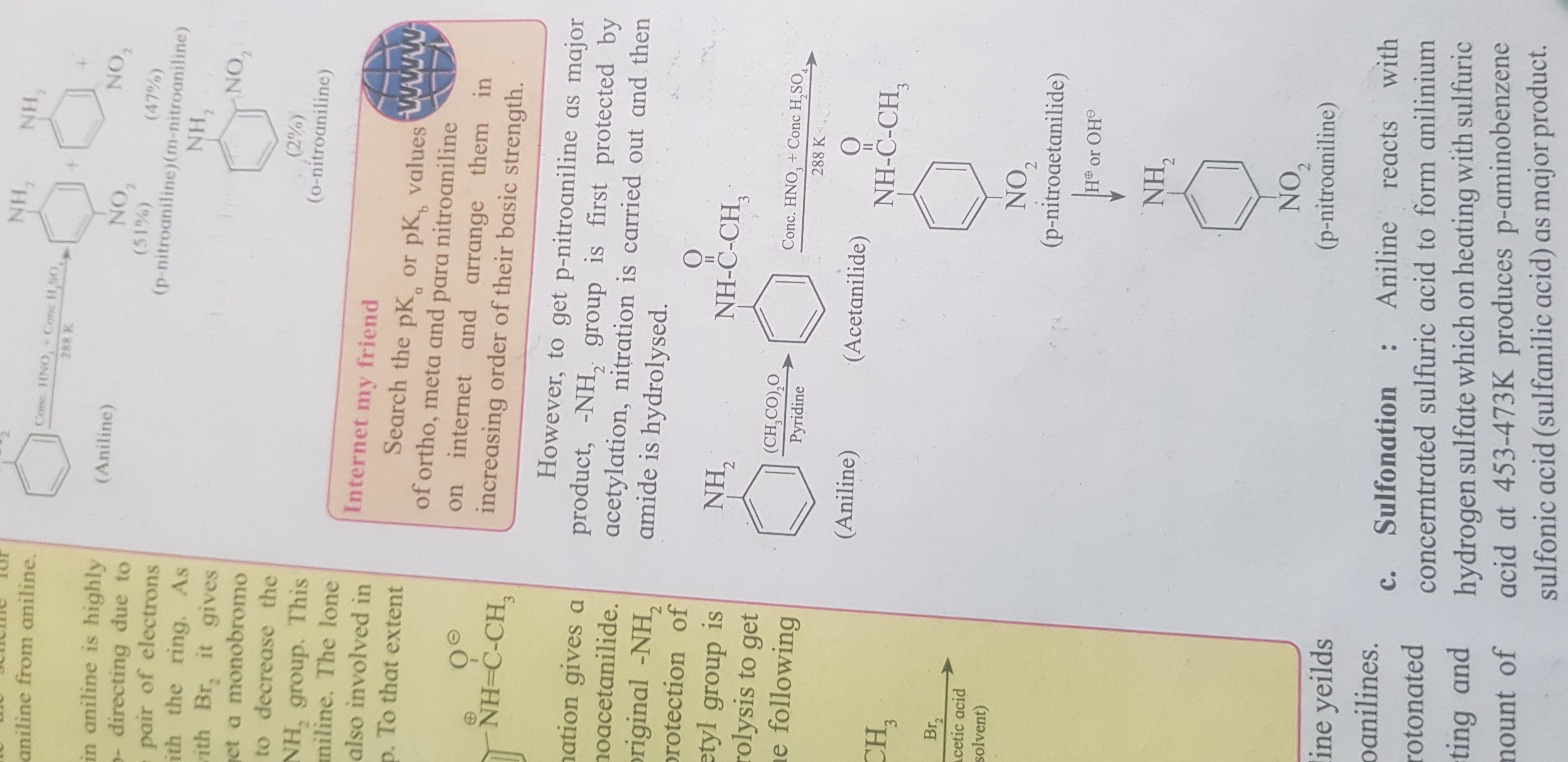
Search the pKa or pKb values of ortho, meta, and para nitroaniline and arrange them in increasing order of their basic strength.
Understand the Problem
The question is asking to research the pKa or pKb values of ortho, meta, and para nitroaniline and arrange them in increasing order of their basic strength. This involves understanding the effects of different substituents on the nitrogen atom's ability to donate a proton or accept one.
Answer
o-nitroaniline < p-nitroaniline < m-nitroaniline
The increasing order of basic strength: o-nitroaniline < p-nitroaniline < m-nitroaniline.
Answer for screen readers
The increasing order of basic strength: o-nitroaniline < p-nitroaniline < m-nitroaniline.
More Information
The placement of the nitro group affects the electron-donating ability of the amino group, with meta nitroaniline being the most basic due to less interference from the nitro group compared to ortho and para positions.
Tips
Ensure to arrange them based on pKb values; lower pKb indicates higher basic strength.
Sources
AI-generated content may contain errors. Please verify critical information