Predict the product(s) of the following reaction: [chemical reaction image]
Understand the Problem
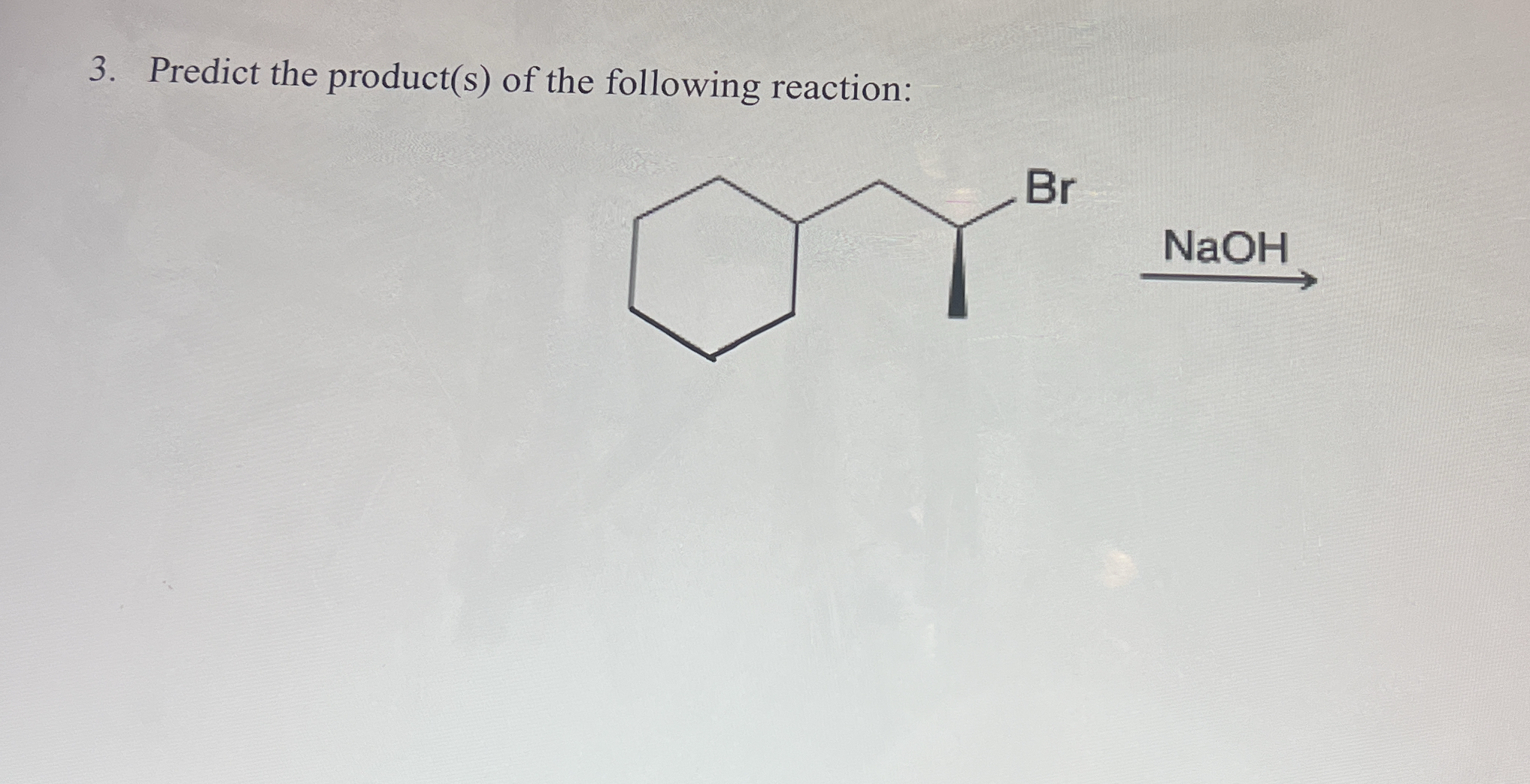
The question asks to predict the product(s) of the given chemical reaction. The reaction involves an alkyl halide (a cyclohexane derivative with a bromine substituent) reacting with NaOH (sodium hydroxide), which is a strong base. This reaction is likely to proceed via either an SN2 or E2 mechanism, depending on the structure of the alkyl halide and the reaction conditions. We need to determine which mechanism is favored and draw the corresponding product(s).
Answer
An E2 reaction occurs, forming a methylcyclohexene.
The reaction of the given cyclohexane derivative with NaOH will result in an elimination reaction (E2), forming an alkene. The major product will be the more stable, more substituted alkene. This will create a cyclohexene derivative with a methyl group.
Answer for screen readers
The reaction of the given cyclohexane derivative with NaOH will result in an elimination reaction (E2), forming an alkene. The major product will be the more stable, more substituted alkene. This will create a cyclohexene derivative with a methyl group.
More Information
NaOH is a strong base and will promote elimination reactions when reacting with alkyl halides. Since the alkyl halide is secondary, it will proceed through an E2 mechanism.
Tips
Make sure to consider all possible products of elimination reactions, and identify the most stable one.
Sources
AI-generated content may contain errors. Please verify critical information