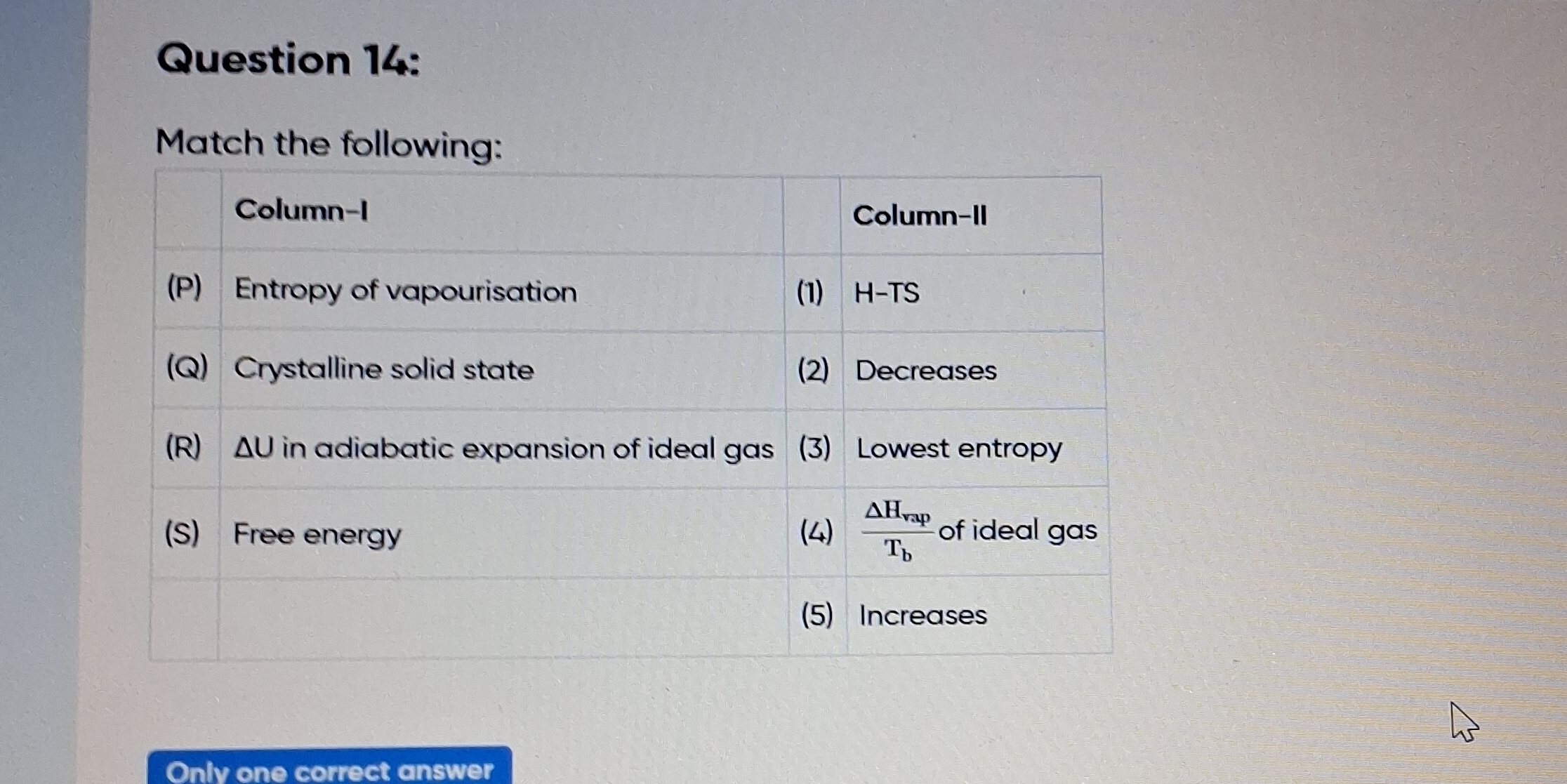
Match the following: (P) Entropy of vapourisation, (Q) Crystalline solid state, (R) ΔU in adiabatic expansion of ideal gas, (S) Free energy.
Understand the Problem
The question is about matching concepts from thermodynamics to their corresponding definitions or effects. It requires knowledge of entropy, phases of matter, and energy changes during processes involving ideal gases.
Answer
(P) - 4, (Q) - 3, (R) - 2, (S) - 1.
The matches are: (P) - 4, (Q) - 3, (R) - 2, (S) - 1.
Answer for screen readers
The matches are: (P) - 4, (Q) - 3, (R) - 2, (S) - 1.
More Information
Entropy of vapourisation is related to the enthalpy of vaporization over the boiling temperature.
Tips
Ensure to link entropy and energy terms correctly, remembering key concepts about physical states and energy changes.
Sources
- Example Source - shaalaa.com
AI-generated content may contain errors. Please verify critical information