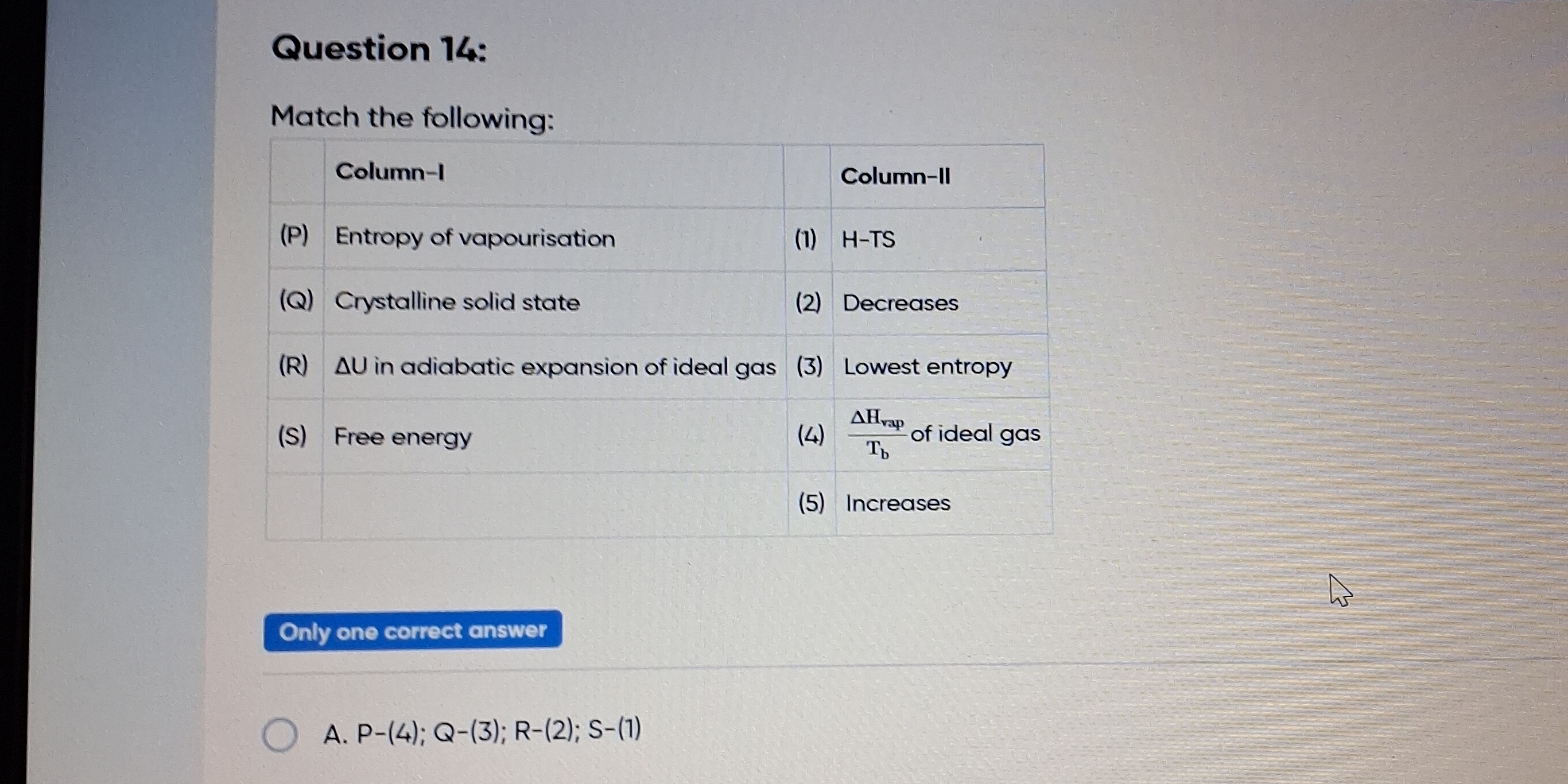
Match the following: Column-I (P) Entropy of vapourisation (Q) Crystalline solid state (R) ΔU in adiabatic expansion of ideal gas (S) Free energy Column-II (1) H-TS (2) Decreases... Match the following: Column-I (P) Entropy of vapourisation (Q) Crystalline solid state (R) ΔU in adiabatic expansion of ideal gas (S) Free energy Column-II (1) H-TS (2) Decreases (3) Lowest entropy (4) ΔH_vap / T_b of ideal gas (5) Increases
Understand the Problem
The question is asking to match items from two columns, relating concepts from thermodynamics and physical chemistry. Specifically, it requires an understanding of vaporisation, states of matter, and changes in energy and entropy.
Answer
P-(4); Q-(3); R-(2); S-(1).
The correct matches are: P-(4); Q-(3); R-(2); S-(1).
Answer for screen readers
The correct matches are: P-(4); Q-(3); R-(2); S-(1).
More Information
Each item is matched based on fundamental thermodynamic principles, such as the relationship between enthalpy change and vaporization for entropy, the characteristic of crystalline structures, and the definition of Gibbs free energy.
Tips
A common mistake is misinterpreting the process for adiabatic expansion, which actually decreases internal energy for an ideal gas.
AI-generated content may contain errors. Please verify critical information