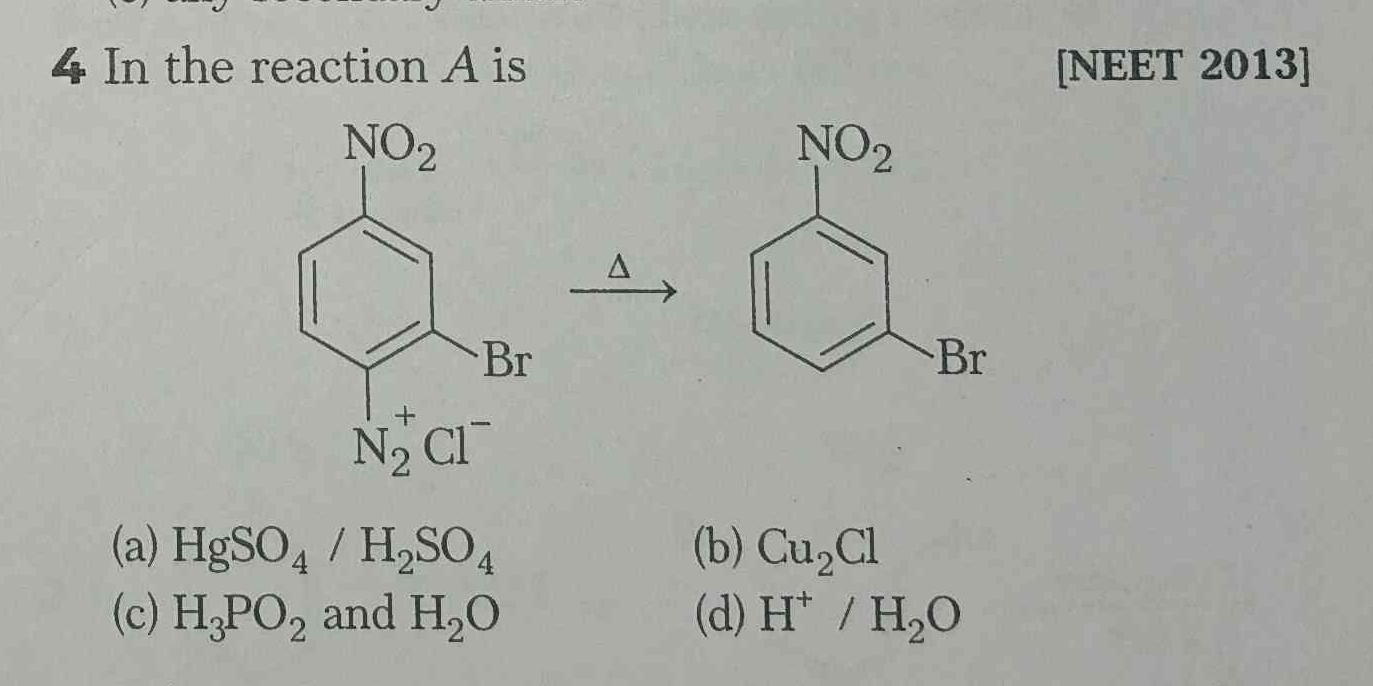
In the reaction A is:
Understand the Problem
The question is asking what reagent 'A' is in the given chemical reaction involving a diazonium compound and the resulting aromatic compound after a specific reaction.
Answer
H3PO2 and H2O
The final answer is H3PO2 and H2O.
Answer for screen readers
The final answer is H3PO2 and H2O.
More Information
In the given reaction, the diazonium group is replaced by a hydrogen atom. H3PO2 and H2O are commonly used for this conversion.
Tips
Ensure you understand that H3PO2 and H2O are used to remove diazonium groups in reactions involving aromatic amines.
Sources
- In the reaction A is - Toppr - toppr.com
- In the reaction, A is - BYJU'S - byjus.com
AI-generated content may contain errors. Please verify critical information