How do you prepare a standard solution of CaCO3?
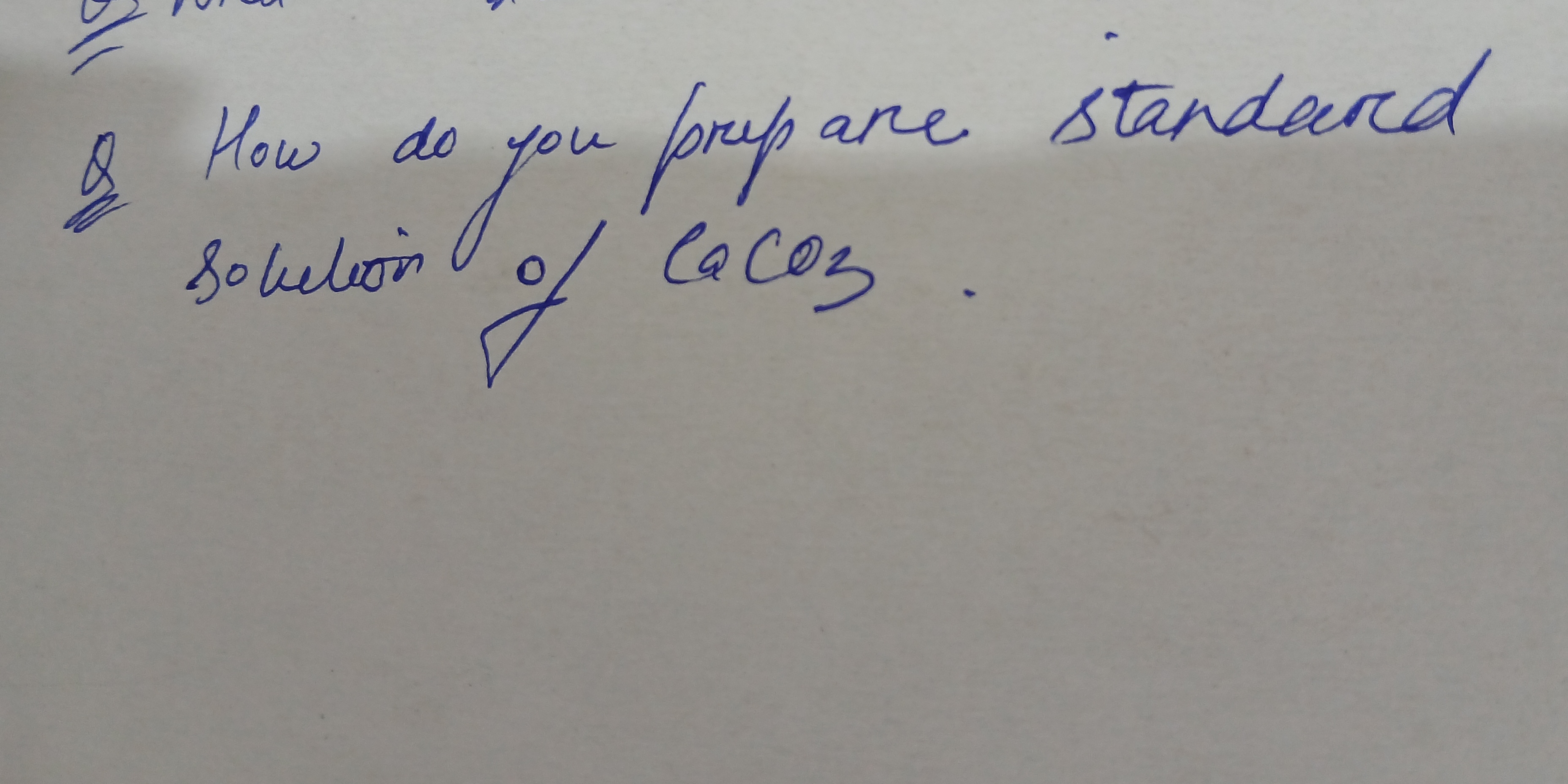
Understand the Problem
The question is asking for the procedure to prepare a standard solution of calcium carbonate (CaCO3). This involves providing the steps and necessary details to ensure accurate preparation.
Answer
Weigh CaCO3, dissolve in water, and dilute to the desired volume.
The final answer is to weigh the desired amount of CaCO3, dissolve it in water, and dilute to the desired volume.
Answer for screen readers
The final answer is to weigh the desired amount of CaCO3, dissolve it in water, and dilute to the desired volume.
More Information
Calcium carbonate is poorly soluble in water; thus, it is typically used in titration settings where precise compatibility is required.
Tips
A common mistake is not dissolving the CaCO3 completely, which can lead to incorrect concentration.
Sources
AI-generated content may contain errors. Please verify critical information