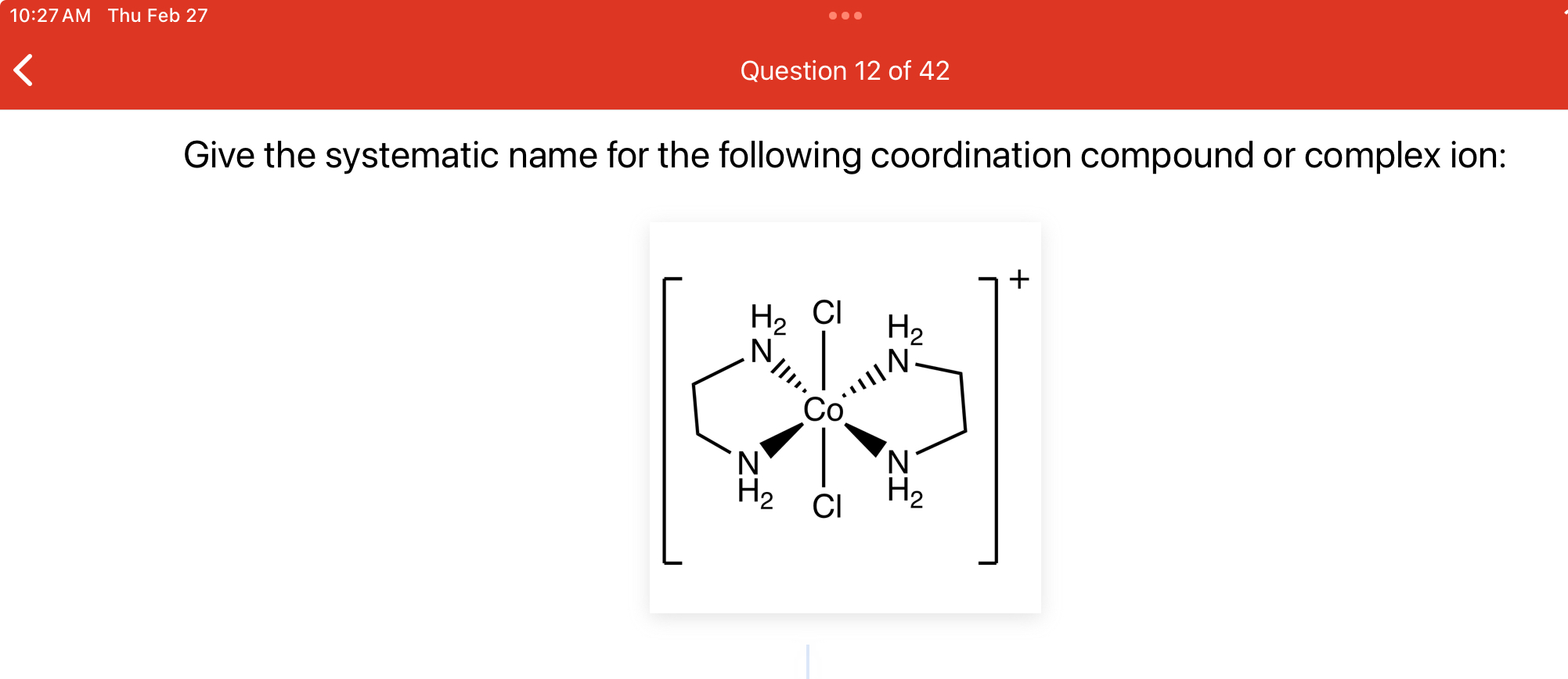
Give the systematic name for the following coordination compound or complex ion:
Understand the Problem
The question asks for the systematic name (IUPAC nomenclature) of a given coordination compound or complex ion. This requires identifying the central metal ion, the ligands, and the overall charge to apply the IUPAC naming conventions correctly.
Answer
dichlorido(ethylenediamine)cobalt(III) ion
The systematic name for the coordination compound is dichlorido(ethylenediamine)cobalt(III) ion.
Answer for screen readers
The systematic name for the coordination compound is dichlorido(ethylenediamine)cobalt(III) ion.
More Information
The compound consists of a central cobalt ion (Co) coordinated by two chloride ligands (chlorido) and one ethylenediamine ligand. Ethylenediamine is a bidentate ligand, meaning it binds to the metal center through two points.
Tips
When naming coordination compounds, remember to name the ligands alphabetically before the metal center. Also, indicate the oxidation state of the metal center using Roman numerals in parentheses.
Sources
- Nomenclature of Coordination Complexes - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information