Find the weight of H₂O in 7 moles.
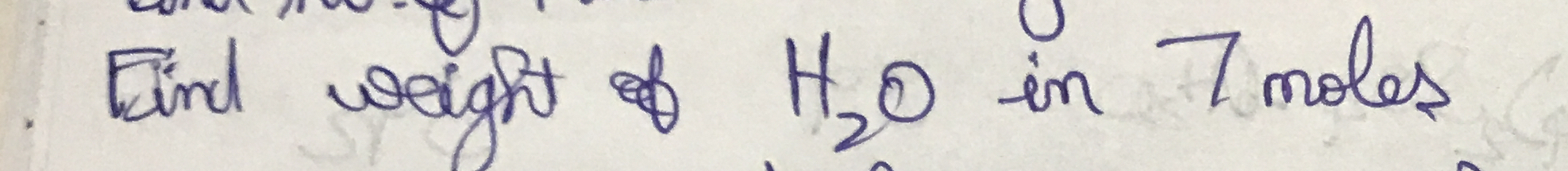
Understand the Problem
The question is asking to find the weight of water (H₂O) in 7 moles. This can be solved by using the molar mass of water to calculate the total weight.
Answer
The weight of H₂O in 7 moles is $126 \text{ g}$.
Answer for screen readers
The weight of H₂O in 7 moles is $126 \text{ g}$.
Steps to Solve
-
Determine the molar mass of water (H₂O)
The molar mass of water can be calculated by adding the atomic masses of its constituent elements: hydrogen (H) and oxygen (O).
- Atomic mass of hydrogen (H) = 1 g/mol. Since there are 2 hydrogen atoms, the total is $2 \times 1 = 2 \text{ g/mol}$.
- Atomic mass of oxygen (O) = 16 g/mol.
Thus, the total molar mass of water is: $$ \text{Molar mass of H₂O} = 2 \text{ g/mol} + 16 \text{ g/mol} = 18 \text{ g/mol} $$
-
Calculate the total weight of water in 7 moles
To find the weight of 7 moles of water, use the formula: $$ \text{Weight} = \text{Moles} \times \text{Molar Mass} $$
Plugging in the values: $$ \text{Weight} = 7 \text{ moles} \times 18 \text{ g/mol} = 126 \text{ g} $$
-
Final Calculation
The calculation shows that the weight of 7 moles of water is: $$ \text{Weight of H₂O} = 126 \text{ g} $$
The weight of H₂O in 7 moles is $126 \text{ g}$.
More Information
Water (H₂O) is essential for life and is a commonly studied substance in chemistry due to its unique properties. Its molar mass at 18 g/mol makes it easy to calculate weights for various amounts in laboratory settings.
Tips
- Forgetting to account for two hydrogen atoms when calculating the molar mass.
- Miscalculating the total weight by not using the correct molar mass or the number of moles.
AI-generated content may contain errors. Please verify critical information