Find the number of particles in 3 moles of N₂.
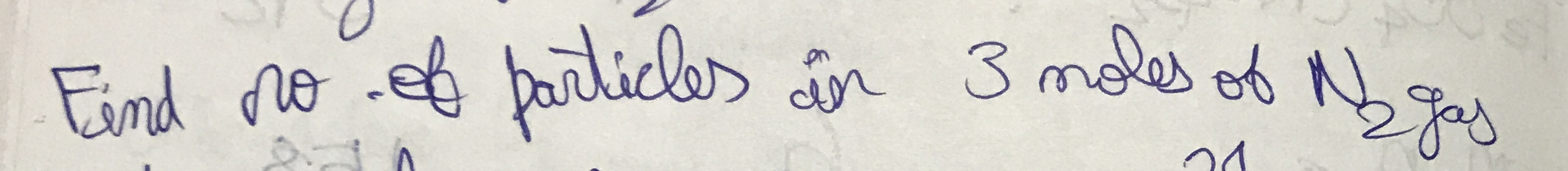
Understand the Problem
The question is asking us to calculate the number of particles in 3 moles of N₂ (nitrogen gas). To solve this, we can use Avogadro's number, which tells us that one mole of any substance contains approximately 6.022 x 10²³ particles.
Answer
The number of particles in 3 moles of N₂ is $1.8066 \times 10^{24}$ particles.
Answer for screen readers
The number of particles in 3 moles of N₂ is approximately $1.8066 \times 10^{24}$ particles.
Steps to Solve
- Identify Avogadro's Number
Avogadro's number states that one mole of any substance contains approximately $6.022 \times 10^{23}$ particles.
- Calculate the Number of Particles in 3 Moles
To find the total number of particles in 3 moles, multiply the number of moles by Avogadro's number: $$ \text{Number of particles} = 3 \text{ moles} \times 6.022 \times 10^{23} \text{ particles/mole} $$
- Perform the Calculation
Now, calculate the product: $$ \text{Number of particles} = 3 \times 6.022 \times 10^{23} = 1.8066 \times 10^{24} \text{ particles} $$
The number of particles in 3 moles of N₂ is approximately $1.8066 \times 10^{24}$ particles.
More Information
This calculation uses Avogadro's number, which is fundamental in chemistry for converting between moles and number of particles. Understanding this concept is essential for stoichiometry and reaction calculations.
Tips
- Forgetting to multiply by Avogadro's number when converting moles to particles.
- Rounding off too early in the calculation, which can lead to inaccuracies.
AI-generated content may contain errors. Please verify critical information