Find mass of 117.2 ml of N2 gas at STP.
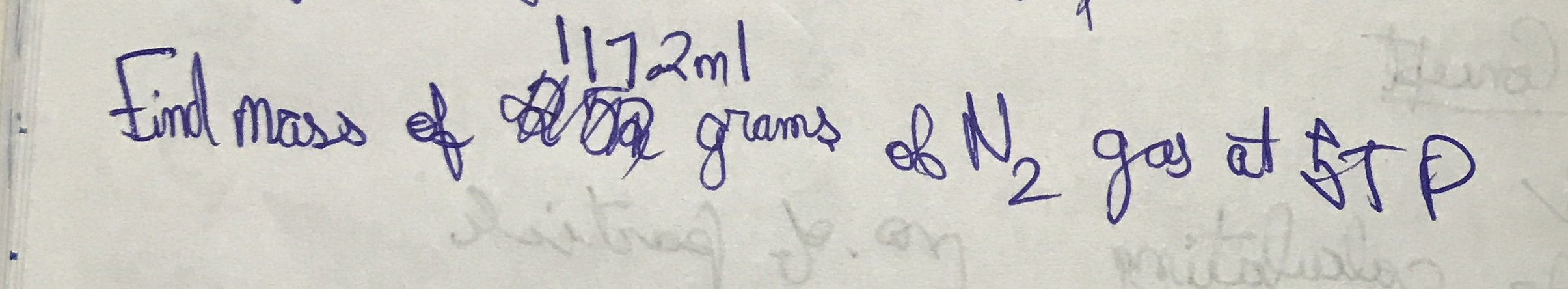
Understand the Problem
The question is asking to calculate the mass of nitrogen gas (N2) in grams, given a volume of 117.2 ml under standard temperature and pressure (STP). To solve this, we can use the ideal gas law and the molar volume of a gas at STP.
Answer
The mass of 117.2 ml of N2 gas at STP is approximately $0.147 \, \text{g}$.
Answer for screen readers
The mass of 117.2 ml of N2 gas at STP is approximately $0.147 , \text{g}$.
Steps to Solve
- Convert volume from milliliters to liters
Since the molar volume at STP (Standard Temperature and Pressure) is typically given in liters, we need to convert the volume: $$ 117.2 , \text{ml} = 0.1172 , \text{L} $$
- Use the ideal gas law to find moles of nitrogen gas (N2)
The molar volume of an ideal gas at STP is approximately $22.4 , \text{L/mol}$. Using this, we can calculate the number of moles ($n$) of N2: $$ n = \frac{\text{Volume}}{\text{Molar Volume}} = \frac{0.1172 , \text{L}}{22.4 , \text{L/mol}} \approx 0.00524 , \text{mol} $$
- Calculate the mass of nitrogen gas
To find the mass, we use the molar mass of N2, which is approximately $28.02 , \text{g/mol}$. Using the number of moles, we then calculate the mass ($m$): $$ m = n \times \text{Molar Mass} = 0.00524 , \text{mol} \times 28.02 , \text{g/mol} \approx 0.147 , \text{g} $$
The mass of 117.2 ml of N2 gas at STP is approximately $0.147 , \text{g}$.
More Information
At standard temperature and pressure (STP), 1 mole of any ideal gas occupies 22.4 liters. This problem used the molar volume to convert the volume of gas into moles, and then the molar mass to find the mass of the gas.
Tips
- Not converting units properly: Make sure to always convert milliliters to liters when using the molar volume at STP.
- Incorrect molar mass usage: Ensure that you use the correct molar mass for nitrogen gas, which is $28.02 , \text{g/mol}$, for $N_2$.
AI-generated content may contain errors. Please verify critical information