Explain alpha, beta, and gamma radiation.
Understand the Problem
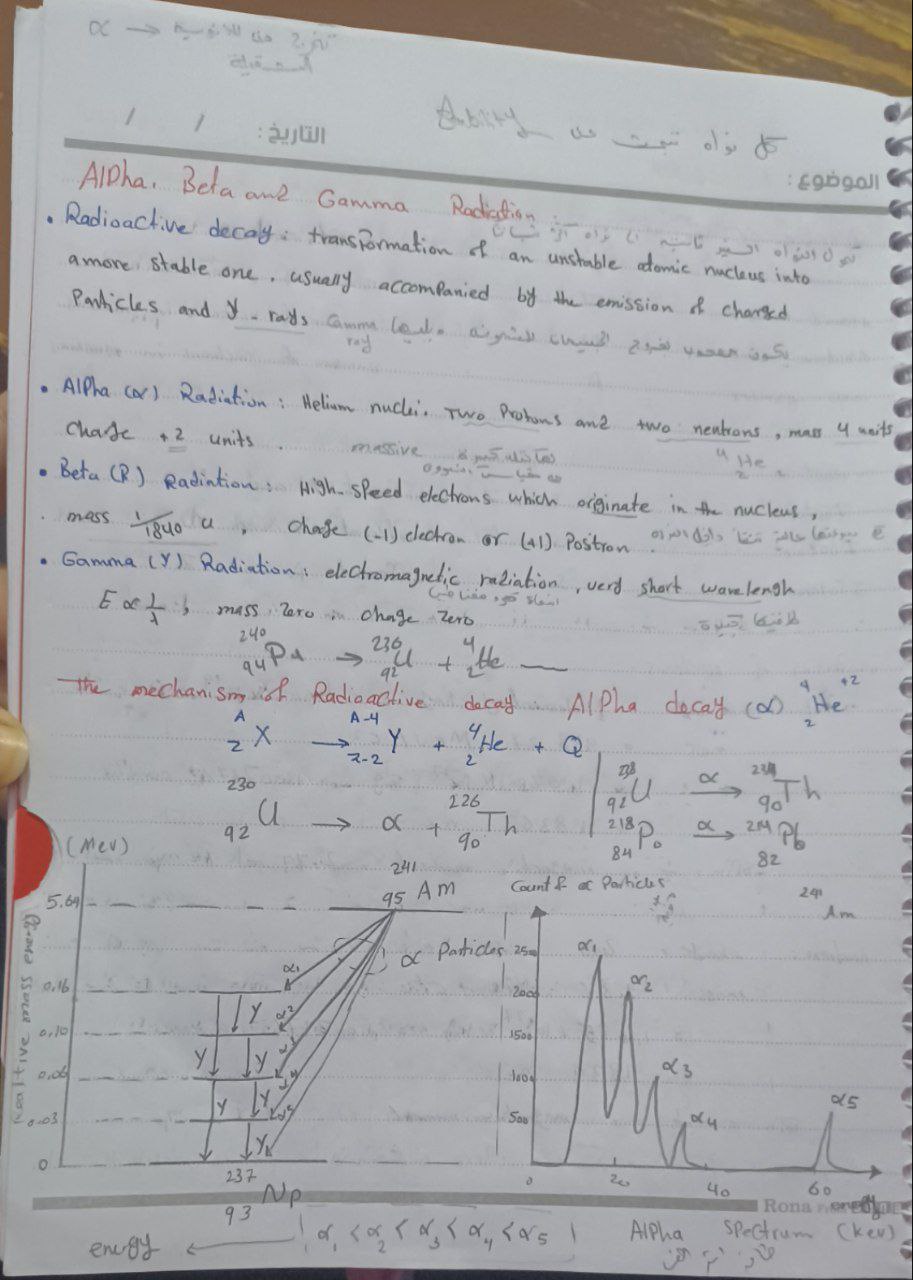
The question relates to radioactive decay processes, specifically focusing on alpha, beta, and gamma radiation. It discusses the characteristics and mechanisms of these forms of radiation and provides illustrative equations and diagrams related to nuclear transformations.
Answer
Alpha: low penetration, +2 charge; Beta: moderate penetration, ±1 charge; Gamma: high penetration, neutral.
Alpha radiation consists of helium nuclei, has a +2 charge, and low penetration. Beta radiation involves high-speed electrons or positrons, can partially penetrate skin, and has a -1 or +1 charge. Gamma radiation is electromagnetic, highly penetrative, and neutral in charge.
Answer for screen readers
Alpha radiation consists of helium nuclei, has a +2 charge, and low penetration. Beta radiation involves high-speed electrons or positrons, can partially penetrate skin, and has a -1 or +1 charge. Gamma radiation is electromagnetic, highly penetrative, and neutral in charge.
More Information
Alpha particles are the least penetrating but most damaging if ingested. Beta particles can penetrate the skin and cause burns. Gamma rays can penetrate most materials, making them highly dangerous without proper shielding.
Tips
A common mistake is confusing the penetration abilities of these radiations. Remember alpha is the least penetrative but most damaging internally.
Sources
- Types of Ionizing Radiation - Mirion Technologies - mirion.com
- Radiation Basics | US EPA - epa.gov
- Properties of Alpha, Beta and Gamma Rays and Differences - BYJU'S - byjus.com
AI-generated content may contain errors. Please verify critical information