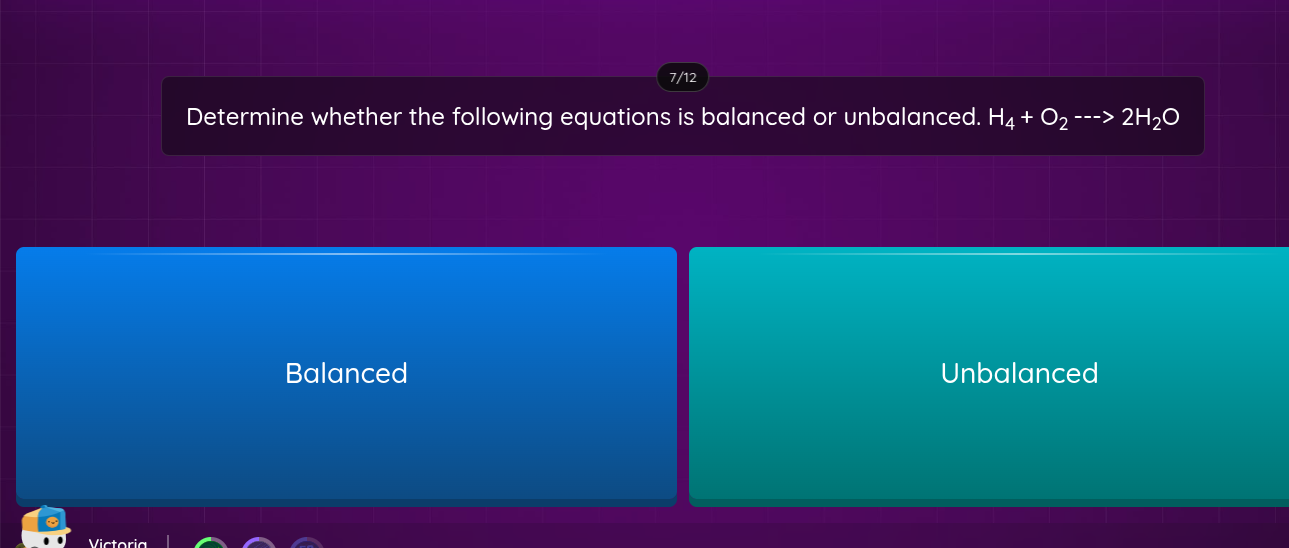
Determine whether the following equation is balanced or unbalanced. H4 + O2 ---> 2H2O
Understand the Problem
The question is asking whether the given chemical equation (H4 + O2 ---> 2H2O) is balanced, meaning that the number of atoms for each element on the reactants side must equal the number on the products side.
Answer
Balanced
The equation H4 + O2 ---> 2H2O is balanced.
Answer for screen readers
The equation H4 + O2 ---> 2H2O is balanced.
More Information
Both sides of the equation have 4 hydrogen atoms and 2 oxygen atoms, making it balanced.
Tips
Ensure each element has the same number of atoms on both sides of the equation.
Sources
- Chemical Equation Balancer - H4 + O2 = H2O - ChemicalAid - chemicalaid.com
AI-generated content may contain errors. Please verify critical information