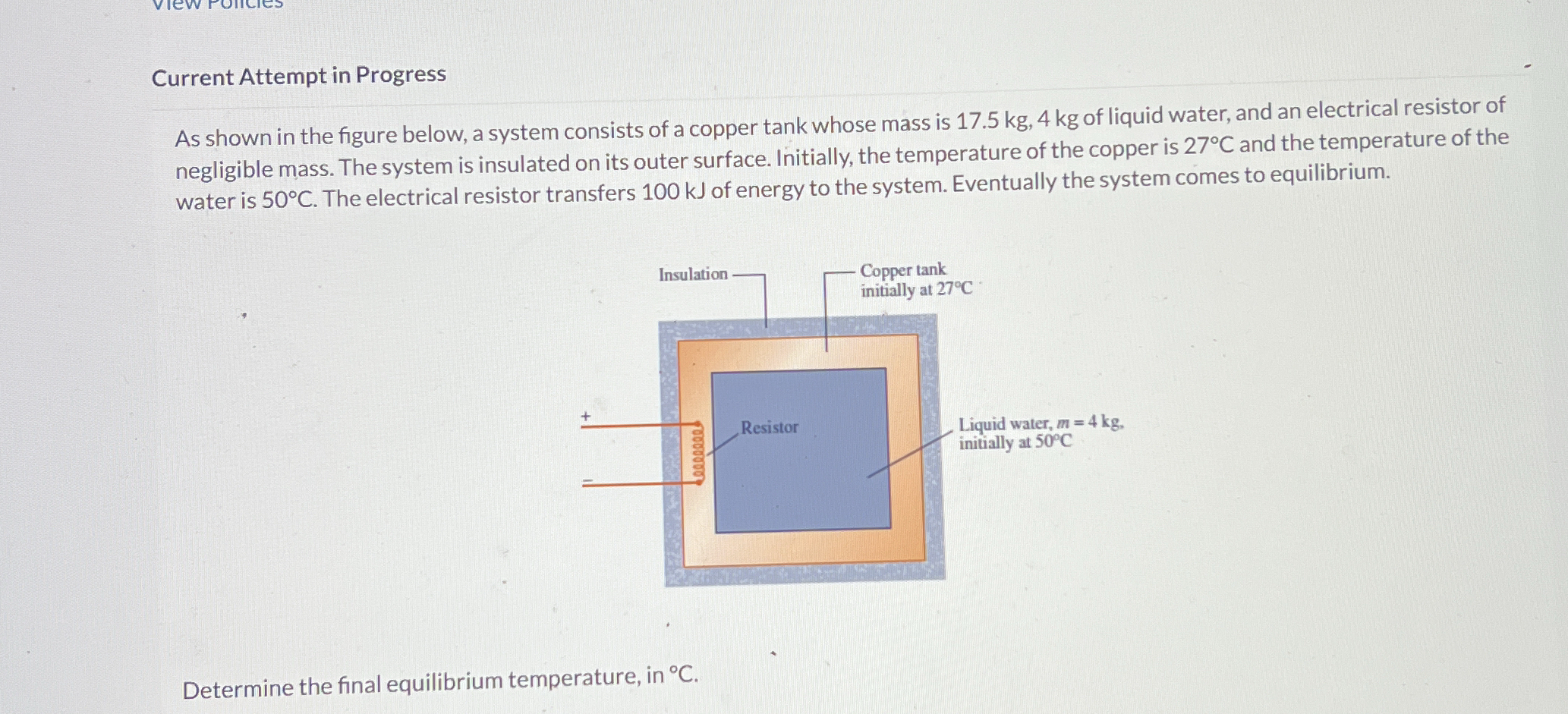
Determine the final equilibrium temperature, in °C. A system consists of a copper tank (mass = 17.5 kg, initial temperature = 27°C), 4 kg of liquid water (initial temperature = 50°... Determine the final equilibrium temperature, in °C. A system consists of a copper tank (mass = 17.5 kg, initial temperature = 27°C), 4 kg of liquid water (initial temperature = 50°C), and an electrical resistor. The system is insulated, and the resistor transfers 100 kJ of energy to the system.
Understand the Problem
The question asks to determine the final equilibrium temperature of a system consisting of a copper tank, liquid water and an electrical resistor. The system is insulated with initial temperatures given for each component. The resistor transfers 100 kJ of energy to the system. We need to calculate the final temperature when the system reaches equilibrium.
Answer
$T_f \approx 47.67^\circ\text{C}$
Answer for screen readers
$T_f \approx 47.67^\circ\text{C}$
Steps to Solve
- Define the system and its components
The system consists of a copper tank, liquid water, and an electrical resistor. The resistor's mass is negligible. We are given:
- Mass of copper, $m_c = 17.5$ kg
- Mass of water, $m_w = 4$ kg
- Initial temperature of copper, $T_{c,i} = 27^\circ$C
- Initial temperature of water, $T_{w,i} = 50^\circ$C
- Energy added by the resistor, $Q = 100$ kJ $= 100,000$ J
- Determine the specific heat capacities
We need the specific heat capacities of copper and water:
- Specific heat of copper, $c_c = 386 , \text{J/kg}^\circ\text{C}$
- Specific heat of water, $c_w = 4186 , \text{J/kg}^\circ\text{C}$
- Apply the energy balance equation
Since the system is insulated, the total energy change is due to the electrical resistor. The energy added by the resistor heats both the copper and the water until they reach a final equilibrium temperature, $T_f$. The change in energy can be written as: $$Q = m_c c_c (T_f - T_{c,i}) + m_w c_w (T_f - T_{w,i})$$
- Solve for the final temperature
Rearrange the equation to solve for $T_f$:
$Q = m_c c_c T_f - m_c c_c T_{c,i} + m_w c_w T_f - m_w c_w T_{w,i}$
$Q + m_c c_c T_{c,i} + m_w c_w T_{w,i} = T_f (m_c c_c + m_w c_w)$
$T_f = \frac{Q + m_c c_c T_{c,i} + m_w c_w T_{w,i}}{m_c c_c + m_w c_w}$
- Plug in the values and calculate $T_f$ $$T_f = \frac{100000 + (17.5)(386)(27) + (4)(4186)(50)}{(17.5)(386) + (4)(4186)}$$
$$T_f = \frac{100000 + 182955 + 837200}{6755 + 16744}$$
$$T_f = \frac{1120155}{23499}$$
$$T_f \approx 47.67^\circ\text{C}$$
$T_f \approx 47.67^\circ\text{C}$
More Information
The final equilibrium temperature of the system is approximately $47.67^\circ\text{C}$. This value lies between the initial temperatures of the copper and water, as expected, and is closer to the initial temperature of water due to its larger mass and specific heat capacity.
Tips
A common mistake is forgetting to convert the energy added by the resistor from kJ to J. Another mistake is using incorrect units for the specific heat capacities or masses. It's also easy to make algebraic errors when rearranging the equation to solve for the final temperature.
AI-generated content may contain errors. Please verify critical information